4576
Clinical utility of intraoperative Arterial Spin Labeling for resection control in brain tumor surgeries, a 3T study.1Radiology, Clínica Universidad de Navarra, Pamplona, Spain, 2IDISNA, Pamplona, Spain, 3Siemens Healthcare, Madrid, Spain, 4Application Development, Siemens Healthcare, Erlangen, Germany, 5Neurosurgery, Clínica Universidad de Navarra, Pamplona, Spain, 6Anesthesia and Intensive Care, Clínica Universidad de Navarra, Pamplona, Spain
Synopsis
ASL has shown potential to depict residual tumor compared to anatomical imaging in previous intraoperative MRI (iMRI) study performed at 1.5T. However, the technique has not been evaluated at higher field, where field inhomogeneities could compromise the labeling efficiency. We aimed to assess feasibility and utility of iMRI-ASL at 3T. To that end, a PCASL sequence was evaluated in 10 patients. In one patient ASL depicted an additional high CBF focus indicating neovascularization (known to correlate with higher grade component) that wasn’t depicted in the anatomical images, favoring the use of ASL in the iMRI setting to achieve maximal resection.
INTRODUCTION
The standard of care for brain tumors, primary or metastatic, dictates maximum safe resection, which is known to improve patients’ progression free survival and quality of life1.The technically improved neurosurgical treatment of brain tumors yields higher rates of complete resection, improving patients’ outcomes. Resection control can be achieved in real time with intraoperative magnetic resonance imaging (iMRI). Available iMRI units up to 3T field strength can provide high quality imaging with optimal spatial and temporal resolution comparable to diagnostic preoperative MRI procedures, allowing the implementation of advanced imaging protocols2. Among the techniques that can be implemented is arterial spin labeling (ASL), a perfusion technique that measures cerebral blood flow (CBF) noninvasively without the use of intravenous contrast agents. ASL provides absolute CBF measurements, of interest in the characterization of tumoral vascularization, which is related to higher grade3.
In a previous study iMRI-ASL was performed at 1.5T field strength, showing clinical potential to depict residual tumor compared to anatomical imaging4,5. However, the technique has not been evaluated at higher field, where issues related to magnetic field inhomogeneities are exacerbated and could reduce the labeling efficiency, compromising quality.
This study aimed to evaluate the feasibility, image quality and potential to depict residual tumor of a pseudo-continuous ASL (PCASL) sequence in the iMRI setting for resection control in patients undergoing brain tumor surgery.
METHODS
Study was approved by the ethics committee. Written informed consent was obtained from all subjects before MRI examination.Inclusion criteria: patients with brain tumoral lesions undergoing resection surgery with iMRI-monitorization for resection control purposes, prospectively recruited. Ten patients met the inclusion criteria.
Prior to surgery, patients underwent a pre-operative MRI study that included ASL.
iMRI setting
The iMRI study was performed in a 3T MRI scanner (MAGNETOM Skyra, Siemens Healthcare, Erlangen, Germany) using two flexible- coils. This equipment is located within the surgical area with direct access to the operating room, with MR-compatible devices. Patients were transferred to the MRI suite when the neurosurgeon judged the resection to be complete or maximally safe.
iMRI protocol
The conventional protocol included precontrast 3D T1-weighted anatomical images (MPRAGE), diffusion images and postcontrast T1-MPRAGE.
A prototype 3D-PCASL sequence6 with a 3D-GRASE readout module, long labeling time (LT) of 3000 ms and post labeling delay (PLD) of 2000 ms was added to the protocol before the injection of exogenous contrast. Acquisition parameters of 3D-PCASL are presented in Figure 1.
CBF maps were computed using the single compartment model7.
Image quality
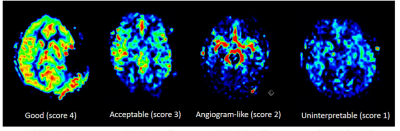
Three independent observers assessed image quality of the resultant ASL-CBF maps using a 4-point scale (4, good; 1, uninterpretable) assisted by example images8. Scores 3-4 were considered of diagnostic quality (Figure 2).
Interobserver agreement was assessed with Fleiss kappa statistics. In cases of disagreement, consensus was reached a posteriori.
Resection control
The datasets considered of diagnostic quality were evaluated by the same observers blindly to assess the presence of residual tumor by both conventional and ASL sequences, using a 3-point rating scale (0, no residual tumor; 1, residual tumor; 2, uncertain diagnosis). In cases of disagreement a consensus was reached. Interobserver agreement was assessed with Fleiss kappa statistics.
CBF ratio calculation
Intraoperative cerebral blood flow ratio (CBFratio) calculated by dividing the CBF measured in the surgical cavity margins or regions of suspected residual tumor by the CBF of the homologous contralateral hemisphere, was compared to preoperative ASL CBFratio (Wilcoxon’s rank-sum test).
RESULTS AND DISCUSSION
Ten patients met the inclusion criteria (seven male and three female: 50.2±17 years [mean±standard deviation], range: 11-76 years).Image quality
Agreement in image quality assessment was moderate (Fleiss κ=0.60). In the subsequent consensus evaluation, diagnostic image quality was observed in 7/10 of the CBF maps. Among the other 3 cases, two CBF maps were scored as 2 (angiogram-like) and one was scored as 1 (uninterpretable).
Resection control
Among the 7 patients with CBF maps of diagnostic quality, the iMRI evaluation with conventional sequences assessed complete resection in four patients, where ASL also determined complete removal.
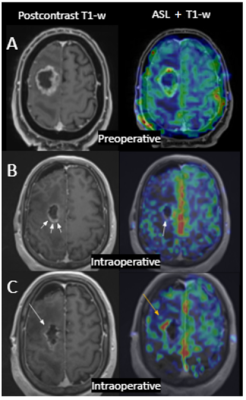
Three patients had residual tumor assessed with the conventional techniques (1 enhancing tumor; 2 non-enhancing lesion). ASL showed an additional focus suspicious of residual tumor in one patient (Figure 3) that was confirmed in the follow-up.
An advantage of perfusion techniques is that they are not influenced by the surgically induced blood-brain barrier breakdown that appears as a lineal enhancement in the surgical margins, that can interfere in the evaluation of residual tumor with the conventional techniques9. Our results are consistent with previous literature evaluating residual component using intraoperative ASL at 1.5T4,5.
Interobserver variability was similar for conventional sequences (to what radiologists are used) (Fleiss κ=0.80), and ASL-maps (Fleiss κ=0.79), highlighting the importance of providing example images/training.
CBF ratio
No significant differences were found between pre and intraoperative CBFratios (1.51±1.19 and 1.31±0.61, [mean±standard deviation] respectively, p=1.00) in the three patients with residual tumor, as expected.
CONCLUSION
ASL is a feasible technique during iMRI at 3T and is useful for the intraoperative assessment of residual tumor, providing additional information to the conventional sequences. This technique can facilitate more aggressive management of highly hyperperfused residual component, potentially leading to associated survival benefits.Acknowledgements
Funding: Spanish Ministry of Science and Innovation (grant: PI18/00084).References
1. Lacroix M, Abi-Said D, Fourney DR, Gokaslan ZL, Shi W, DeMonte F, Lang FF, McCutcheon IE, Hassenbusch SJ, Holland E, Hess K, Michael C, Miller D, Sawaya R. A multivariate analysis of 416 patients with glioblastoma multiforme: prognosis, extent of resection, and survival. J Neurosurg. 2001 Aug;95(2):190-8. doi: 10.3171/jns.2001.95.2.0190. PMID: 11780887.
2. Rogers CM, Jones PS, Weinberg JS. Intraoperative MRI for Brain Tumors. J Neurooncol. 2021 Feb;151(3):479-490. doi: 10.1007/s11060-020-03667-6. Epub 2021 Feb 21. PMID: 33611714.
3. Soldozy S, Galindo J, Snyder H, Ali Y, Norat P, Yağmurlu K, et al. Clinical utility of arterial spin labeling imaging in disorders of the nervous system. Neurosurg Focus. 2019;47(6):1–10.
4. Lindner T, Ahmeti H, Lübbing I, Helle M, Jansen O, Synowitz M, Ulmer S. Intraoperative resection control using arterial spin labeling - Proof of concept, reproducibility of data and initial results. Neuroimage Clin. 2017 Apr 25;15:136-142. doi: 10.1016/j.nicl.2017.04.021. PMID: 28507896; PMCID: PMC5423346.
5. Lindner T, Ahmeti H, Juhasz J, Helle M, Jansen O, Synowitz M, Ulmer S. A comparison of arterial spin labeling and dynamic susceptibility perfusion imaging for resection control in glioblastoma surgery. Oncotarget. 2018 Apr 6;9(26):18570-18577. doi: 10.18632/oncotarget.24970. PMID: 29719627; PMCID: PMC5915094.
6. Vidorreta M, Wang Z, Rodríguez I, Pastor MA, Detre JA, Fernández-Seara MA. Comparison of 2D and 3D single-shot ASL perfusion fMRI sequences. Neuroimage. 2013 Feb 1;66:662-71. doi: 10.1016/j.neuroimage.2012.10.087. Epub 2012 Nov 7. PMID: 23142069; PMCID: PMC3587033.
7. Alsop DC, Detre JA, Golay X, Günther M, Hendrikse J, Hernandez-Garcia L, Lu H, MacIntosh BJ, Parkes LM, Smits M, van Osch MJ, Wang DJ, Wong EC, Zaharchuk G. Recommended implementation of arterial spin-labeled perfusion MRI for clinical applications: A consensus of the ISMRM perfusion study group and the European consortium for ASL in dementia. Magn Reson Med. 2015 Jan;73(1):102-16. doi: 10.1002/mrm.25197. Epub 2014 Apr 8. PMID: 24715426; PMCID: PMC4190138.
8. Ferro DA, Mutsaerts HJ, Hilal S, Kuijf HJ, Petersen ET, Petr J, van Veluw SJ, Venketasubramanian N, Yeow TB, Biessels GJ, Chen C. Cortical microinfarcts in memory clinic patients are associated with reduced cerebral perfusion. J Cereb Blood Flow Metab. 2020 Sep;40(9):1869-1878. doi: 10.1177/0271678X19877403. Epub 2019 Sep 26. PMID: 31558107; PMCID: PMC7430096.
9. Ulmer S, Helle M, Jansen O, Mehdorn HM, Nabavi A. Intraoperative dynamic susceptibility contrast weighted magnetic resonance imaging (iDSC-MRI) - Technical considerations and feasibility. Neuroimage. 2009 Mar 1;45(1):38-43. doi: 10.1016/j.neuroimage.2008.11.021. Epub 2008 Dec 6. PMID: 19100843.
Figures