4571
High resolution myocardial T1mapping with a deep learning constrained Compressed SENSE reconstruction1radiology, Tokyo metropolitan police hospital, Tokyo, Japan, 2Tokyo metropolitan police hospital, Tokyo, Japan, 3Philips Japan, Tokyo, Japan, 4Philips Healthcare, Best, Netherlands
Synopsis
High-resolution imaging and T1 mapping is needed to achieve useful clinical information optimally in cardiac MRI. However, prolonged acquisition time can lead to poor or non-diagnostic image quality. In this study, we investigated the use of a deep learning-based reconstruction algorithm to highly accelerate T1map acquisition for cardiac MRI. Adaptive-CS-Net, a deep neural network previously introduced at the 2019 fastMRI challenge, was expanded and integrated into the Compressed-SENSE Artificial Intelligence (CS-AI) reconstruction. The purpose of this study was to compare the image quality of high-resolution T1map between reference and accelerated methods: SENSE, Compressed-SENSE, and CS-AI.
Introduction
Cardiac Magnetic Resonance Imaging (CMRI) can provide a lot of clinically useful information such as coronary artery structure, myocardial wall motion, and quantitative measurement of myocardial T1map. High-resolution (HR) imaging, and T1 mapping for the latter, is needed to achieve that optimally. However, prolonged acquisition time can lead to poor or non-diagnostic image quality1-3. Historically, sensitivity encoding (SENSE) has been shown to accelerate the scan while maintaining the image quality. Moreover, Compressed-SENSE (C-SENSE) can shorten the acquisition time further by performing optimized random data under-sampling. However, the residual noise in the image can still become a problem even though denoising in the Wavelet transform domain and iterative reconstruction are used. In this study, Compressed SENSE AI (CS-AI) reconstruction4-5 was used to reduce the noise while preserving the image quality of HR T1 mapping of the right ventricle. It is hypothesized that the image quality of the source image in T1 mapping can be significantly improved by using the CS-AI reconstruction algorithm, thus leading to more reliable diagnostic T1 maps. The purpose of this study was to acquire HR T1 map of the right ventricle using the CS-AI reconstruction and compare the image quality with SENSE and conventional Compressed-SENSE (C-SENSE).Methods
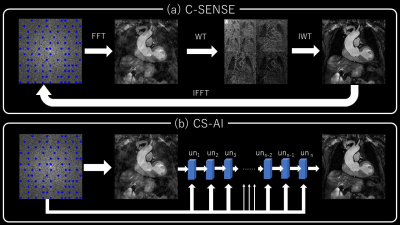
Using our institutional review board-approved procedures, 6 healthy volunteers underwent volunteer examination using a 3.0T Philips MR system and dStream Torso coil. T1mapping sequences were set to short axis orientation including papillary muscle using vector cardiac gating with the following parameters: 2D balanced turbo field echo, FOV (mm) = 300×300, acquisition (mm) = 2×2(Norm)/ 1×1(FR), slice thickness (mm) = 10mm, TR/TE (ms) = 2.9/1.33, SENSE/C-SENSE acceleration factor = 3(Norm)/6(HR), number of signals averaged (NSA) = 1, scan time= 11.4~12.6 (sec: depends on heart beat). The T1map was computed from images reconstructed by SENSE and C-SENSE (denoising level=weak, medium, and strong) and by CS-AI (denoising level=weak, medium, and strong). Images were assessed by using co-efficient of variation (CV%), measured by left ventricle myocardium (LVM) T1 value and standard deviation (SD). In the CS-AI reconstruction, the C-SENSE reconstruction chain is replaced by a convolution neural network (CNN) reconstruction. To be concise, the iterative part of SENSE and wavelet constraining is replaced by a chain of U-Net blocks that perform the transformation from raw into image data. Each U-Net block is fed with the k-space data by which the analogy with the C-SENSE iterative reconstruction scheme exists. This is visually represented in figure 1.Results and Discussions
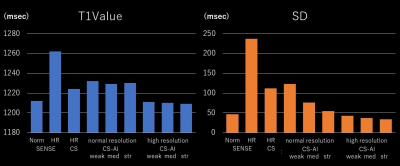
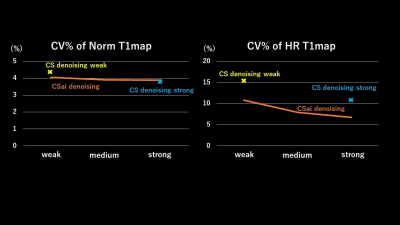
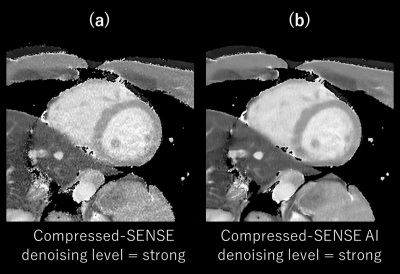
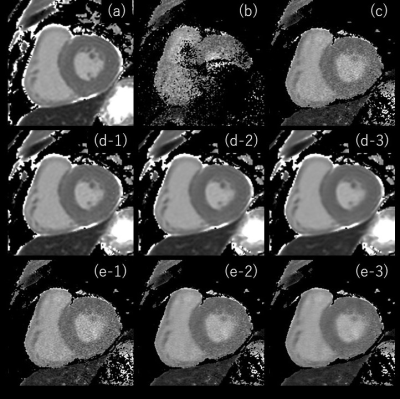
Fig. 2 shows the T1 value and SD of SENSE, C-SENSE, and CS-AI with normal and high-resolutions (HR). The HR CS-AI showed T1 value smaller than the normal resolution CS-AI and similar to the SENSE but with much smaller SD. The SD in CS-AI decreased with stronger denoising and this trend was more significant in normal resolution T1 mapping. The SD in HR CS-AI was smaller than that of SENSE with normal resolution. Fig. 3 shows the CV% of normal and HR T1maps calculated from the image reconstructed by CS-AI. The CV% in the normal resolution did not change significantly depending on the denoising level. However, in HR the SENSE T1map was improved (Fig2-3). Moreover, the high resolution T1map by SENSE could not be used in our analysis due to SENSE aliasing artifact (fig4). Fig. 4 shows the comparison of HR T1 maps reconstructed C-SENSE and CS-AI. The T1 map became very noisy in SENSE and was not suitable for diagnosis. Both C-SENSE and CS-AI visualized overall structures, but small structures were better depicted in CS-AI. Fig. 5 shows the comparison of normal and high resolution T1 maps for SENSE, C-SENSE, and CS-AI. On normal resolution with acceleration factor 3, both SENSE (a) and C-SENSE (d) produced T1 maps with visually good quality. The denoising level did not significantly influence the overall T1 map quality on normal resolution C-SENSE (d). However, on HR T1 maps with acceleration factor 6, the T1 map in C-SENSE became very noisy (c). On CS-AI (e), the quality of T1map image significantly improved as the denoising level increased from weak to strong. With the denoising level strong (e-3), small difference of the contrast depending on the myocardial position could be recognized due to the improved T1 map quality.Conclusion
The use of CS-AI reconstruction could improve the image quality of high resolution T1 map compared to SENSE and C-SENSE, allowing a spatial resolution for the T1 maps not attainable before in a clinical setting.Acknowledgements
No acknowledgement found.References
1. Nordio, G., Henningsson, M., Chiribiri, A., Villa, A. D., Schneider, T., & Botnar, R. M. (2017). 3D myocardial T1 mapping using saturation recovery. Journal of Magnetic Resonance Imaging, 46(1), 218-227.
2. Kirsten MB, Jeanette SM, Tobias S, et al. high‐resolution cardiac T 1 mapping and cine imaging using model‐based iterative image reconstruction. Magn Reson Med . 2019;81:1080–1091.
3. Mehta BB, Gonzalez JA, Salerno M, et al. High-resolution T1 mapping with ANGIE detects increased right-ventricular extracellular volume fraction in patients with pulmonary arterial hypertension. Journal of Cardiovascular Magnetic Resonance 17.S1 (2015): O39.
4. Pezzotti N, Yousefi S, Elmahdy MS, et al. An Adaptive Intelligence Algorithm for Undersampled Knee MRI Reconstruction. IEEE Access. 2020;8:204825-204838.
5. Pezzotti N, de Weerdt E, Yousefi S, et al. Adaptive-CS-Net: FastMRI with Adaptive Intelligence. arxiv. 2019;(NeurIPS).
Figures