4556
Assessments of hemodynamic changes in abdominal aortic aneurysm in patients using 4D EPI flow MRI1West China Hospital, Sichuan University, Chengdu, China, 2Clinical Science, Philips Healthcare, Chengdu, China, Chengdu, China
Synopsis
Four-dimensional flow MRI (4D flow MRI) plays a significant role in cardiovascular imaging, which provides new insights into the pathophysiology of disease. In this study, 4D flow with echo-planar imaging was introduced to evaluate the hemodynamic parameters of patients with abdominal aortic aneurysm, including velocity, flow and wall shear stress. We propose that 4D EPI flow can find differences of the above parameters between abdominal aortic aneurysm and normal abdominal aorta and has potential to be used for hemodynamics assessment in AAA patients.
Introduction
Abdominal aortic aneurysm (AAA) is a common disease caused by a weakening in the aortic wall and found about one in twenty men over the age of 65[1]. Probability of vessel rupture increases with the diameter of AAA[2].However, the diameter is not representative the rupture risk of aneurysm, and there is still a lack of comprehensive and clinically relevant data on the hemodynamics of AAA.Four-dimensional flow MRI (4D flow MRI)[3] could be used to analyze complex hemodynamic models in vivo by quantifying blood flow parameters and deriving characteristics, and to offer direct measurement of advanced indicators like wall shear stress (WSS), providing new insights into disease pathophysiology. However, integrating 4D flow MRI in routine abdominal protocols has been challenging, in part due to long scan times. Recently, 4D flow with echo-planar imaging (4D EPI flow) has emerged as a novel technique to offer additional acceleration on top of parallel imaging, which can enable the comprehensive evaluation of complex blood flow[4]. However, validation of this technique for AAA evaluation is needed for both clinical and research applications. Therefore, the aim of the present study was to validate 4D EPI flow MRI for visualizing and quantifying the hemodynamics of AAA.
Methods
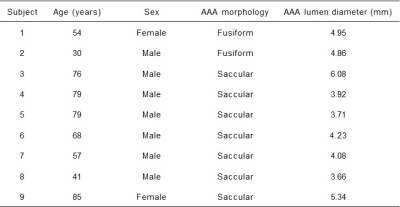
9 AAA patients (7 males, 2 females; aged 63.22±18.97 years) were recruited in this study, with the details shown in Table 1. Informed consent was obtained from all subjects before MR scanning on a clinical 3T system (Elition, Philips Healthcare, Best, the Netherlands), equipped with a 32-channel body coil.Standard 2D PC MRI was performed at lower renal artery opening, which served as a reference for comparison with 4D flow MRI. Free breathing 4D EPI flow was performed with the scan parameters listed as follows: EPI factor =5, flip angle =10o, TR/TE= 8.0/4.1 msec, FOV=173×315×380mm3 , with acquired and reconstructed spatial resolutions were 2.5×2.5×2.5 and 1.98×1.98×2.0mm3. Three-directional velocity-encoding sensitivity (VENC) was set to 100-300 cm/second, depending on the presence of velocity aliasing in 2D flow MRI scout images.
Image Analysis
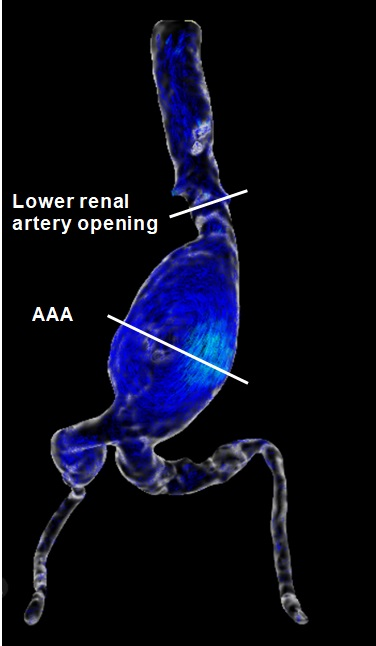
Post-analysis of 4D flow data were performed with the Circle Cardiovascular Imaging (CVI42, Canada). Two analysis planes, transecting the aortic lumen orthogonal to the anticipated main flow direction, were manually placed in lower renal artery opening (normal abdominal aorta) and AAA level regions (Fig. 1). The quantitative measured parameters included: average through-plane velocity, peak velocity, average net flow, peak flow, and average circumferential WSS in each plane.
Statistical analysis
Continuous variables were reported as means ± standard deviations. The paired t-test was used for statistically analyzing quantitative measured parameters with SPSS (V21.0A, IBM Corporation, Armonk, NY, USA). A p-value of less than 0.05 was considered as a significant difference.
Results and Discussion
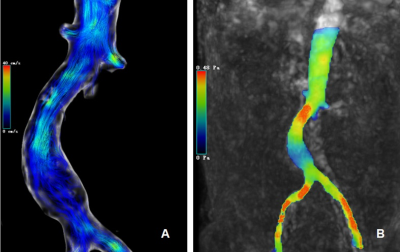
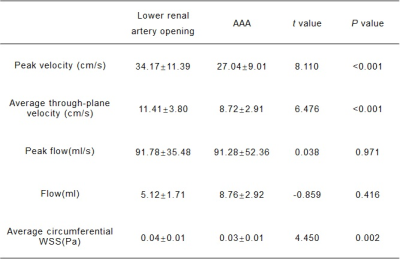
VelocityThe peak velocity between lower renal artery opening and AAA showed significant difference (34.17±11.39 vs 27.04±9.01, P<0.001). The average through-plane velocity between lower renal artery opening and AAA also showed significant difference (11.41±3.80 vs 8.72±2.91, P<0.001) (Table 2 & Fig. 2A).
Flow
The peak flow and average net flow differ slightly between the normal aorta and AAA, however, there was no significant difference, maybe in part due to current relatively small number of subjects included in this study ( peak flow: 91.78±35.48 vs 91.28±52.36, P=0.971; average net flow: 5.12±1.71 vs 8.76±2.92, P=0.416).
WSS
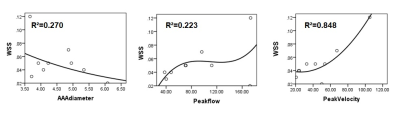
The significant WSS changes were noted in patients with AAA. Compared to lower renal artery opening, average circumferential WSS decreased significantly in abdominal aortic aneurysms (P=0.002) (Fig. 2B). The correlation between WSS and various parameters was also analyzed. There was a weak negative correlation between WSS and AAA diameter (r=-0.460), a weak positive correlation with peak flow (R =0.444), and a strong positive correlation with peak velocity (R =0.857), and appropriate fitting curves were used to represent them respectively (Fig. 3). Recent years, most studies believe that low WSS has the highest correlation with AAA rupture, which indicates that low WSS is not only related to the generation of thrombosis, but also may directly cause rupture due to irreversible vascular injury[5]. A systematic review and meta-analysis[6] found that WSS was significantly higher in symptomatic or ruptured AAA patients than in asymptomatic AAA patients. However, there is still uncertainty about measuring WSS values for predicting AAA ruptures[7].
Conclusion
In this study, 4D EPI flow MRI was used for evaluation of changes in hemodynamic parameters in patients with AAA. In the abdominal aortic aneurysm, there was no significant change in peak flow and average net flow, however, both flow rate and WSS decreased when compared to normal aorta, In addition, WSS had a certain correlation with AAA diameter, flow and velocity, which may be associated with the growth, rupture and thrombosis of abdominal aortic aneurysm. A large clinical study is underway to validate the diagnostic value of this technique.Acknowledgements
No acknowledgement found.References
1. Sampson, U.K., et al., Global and regional burden of aortic dissection and aneurysms: mortality trends in 21 world regions, 1990 to 2010. Glob Heart, 2014. 9(1): p. 171-180 e10.
2. Lederle, F.A., et al., Rupture rate of large abdominal aortic aneurysms in patients refusing or unfit for elective repair. JAMA, 2002. 287(22): p. 2968-72.
3. Dyverfeldt, P., et al., 4D flow cardiovascular magnetic resonance consensus statement. J Cardiovasc Magn Reson, 2015. 17: p. 72.
4. Garg, P., et al., Comparison of fast acquisition strategies in whole-heart four-dimensional flow cardiac MR: Two-center, 1.5 Tesla, phantom and in vivo validation study. J Magn Reson Imaging, 2018. 47(1): p. 272-281.
5. Boyd, A.J., et al., Low wall shear stress predominates at sites of abdominal aortic aneurysm rupture. J Vasc Surg, 2016. 63(6): p. 1613-9.
6. Khosla, S., et al., Meta-analysis of peak wall stress in ruptured, symptomatic and intact abdominal aortic aneurysms. Br J Surg, 2014. 101(11): p. 1350-7; discussion 1357.
7. Indrakusuma, R., et al., Biomechanical Imaging Markers as Predictors of Abdominal Aortic Aneurysm Growth or Rupture: A Systematic Review. Eur J Vasc Endovasc Surg, 2016. 52(4): p. 475-486.
Figures