4553
Association of Aortic Wall Characteristics and Cardiac Function in Fontan Patient1Department of Biomedical Engineering and Environmental Sciences, National Tsing Hua University, Hsinchu, Taiwan, 2Department of Radiology, Kaohsiung Veterans General Hospital, Kaohsiung, Taiwan, 3Department of Pediatrics, Kaohsiung Veterans General Hospital, Kaohsiung, Taiwan
Synopsis
The interaction of aortic wall characteristics and cardiac function in Fontan patients is unclear. 4D flow MRI was employed to quantify the wall shear stress (WSS) and oscillatory shear index (OSI) in the aorta. Fontan patients exhibited increased axial WSS, decreased axial OSI and non-roundness of WSS in descending aorta (DAo). We also found that the increased axial WSS in DAo correlated with decreased ejection fraction and increased indexed end-systolic volume. The cardiac dysfunction presented adverse interaction with aortic wall characteristics in patients with Fontan, especially in DAo, and might lead to higher risk of atherosclerosis and stenosis.
Introduction
The Fontan procedure is a palliative surgery used in children with functional single ventricle 1. Previous studies reported cardiac dysfunction, such as ventricular failure, thromboembolism, and arrhythmias 2,3, in Fontan patients. In addition to the function of the single ventricle, the aorta is also vital to withstand the blood from the single ventricle in Fontan patients. Abnormal aortic wall characteristics reflected the endothelial cells dysfunction and lower vasoactive function 4. Cardiac dysfunction was found in patients with aortic wall stiffness in previous study 5. However, the interaction of aortic wall characteristics and cardiac function in Fontan patients is unclear. In this study, we characterized aortic wall with measurements of aortic wall shear stress (WSS) and oscillatory shear index (OSI) by 4D flow MRI. The purpose of this study was to evaluate the association of aortic wall characteristics and cardiac function in patients after the Fontan operation.Methods
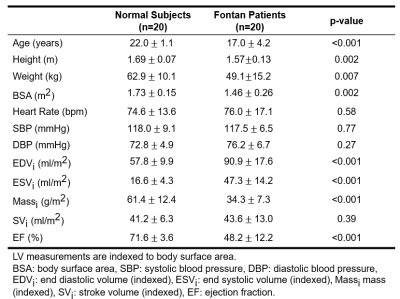
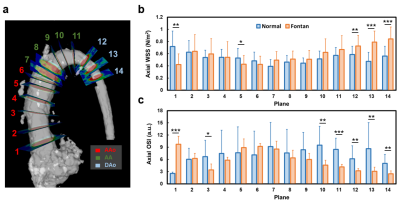
This study recruited 20 Fontan patients (17.0±4.2 y/o, 9 males, 11 females) and 20 age-appropriate normal volunteers (22.0±1.1 y/o, 13 males, 7 females). The 4D flow MRI was performed on a 3.0-Tesla MR scanner (Trio with Tim or Skyra, Siemens, Erlangen, Germany). 4D flow MRI was performed with prospective ECG trigging, navigator-guided free-breathing technique, and scanning parameters of TR= 5.5ms, TE= 2.57ms, flip angle=7°, voxel size= 2.2x 1.5x3.5 mm3, Venc=150 cm/s. Denoising, anti-aliasing and reconstruction were applied to 4D flow data. The 14 planes was prescribed along the long-axis of the aorta with landmarks of sinuses of Valsalva, the level of pulmonary artery bifurcation and the brachiocephalic arteries, as shown in Figure 1(a). The axial WSS (τ) was calculated by 6: $$\tau = \mu\frac{\partial u(x,y)}{\partial x\partial y}$$ Where μ is the viscosity 0.0035 N∙s/m2, u is the profile of the blood flow velocity. τ is equal to slope in geometry. A non-roundness (NR) of WSS was used to present the distribution of WSS along the circumference of each plane. The NR was calculated by 7: $$NR=\sqrt{{\frac{1}{N}}\sum_{i=1}^N(\tau_{i}-\bar{\tau})^{2}}/\bar{\tau}$$ Where N is equal to 12. The axial OSI was defined as 6: $$OSI = \frac{1}{2}(1-\frac{|\int_{0}^{T}\tau\cdot dt|}{\int_{0}^{T}|\tau|\cdot dt})$$ Where τ is the axial WSS and T is the duration of a cardiac cycle. The Mann-Whitney U test was used to compare the differences between Fontan patients and normal controls. Pearson correlation was employed to associate cardiac function and vessel wall characteristics. A p < 0.05 was considered as statistical significance.Results
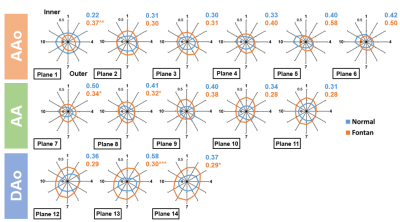
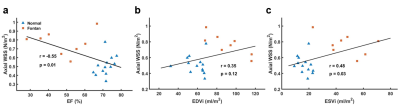
Table 1 illustrates the demographics and cardiac MRI volumetric parameters. Figures 1(b,c) show the peak axial WSS and OSI in normal controls and Fontan patients. In comparison to normal group, Fontan group presented lower axial WSS and higher OSI in the aortic root (p<0.01~0.001) while showed significantly higher axial WSS and lower OSI in descending aorta (DAo) (p<0.01~0.001). As shown in Figure 2, Fontan group presented lower NR in AA and DAo (p<0.05~0.001) than that in normal group. However, the higher NR was observed in AAo (p<0.01), especially in the aortic root. Figure 3 shows the correlation of axial WSS in DAo and the volumetric parameters. Negative correlation was observed in axial WSS and EF (r=-0.55, p=0.01) while positive correlations were shown in axial WSS and indexed end-systolic volume (ESVi) (r=0.48, p=0.03).Discussion and Conclusions
In this study, Fontan patients exhibited increased axial WSS, decreased axial OSI and NR of WSS in DAo. We also found that the increased axial WSS in DAo correlated with decreased EF and increased ESVi. Previous study suggested that higher WSS reflected endothelial cell dysfunction and decreased vasoactive in the in vivo situation [4]. Decreased OSI was observed around coronary stenosis [8]. In this study, the abnormal WSS and OSI were observed in Fontan patients. Besides, decreased NR of WSS in the DAo of Fontan patients illustrated the low eccentricity of WSS distribution along the circumference of DAo. The altered aortic wall characteristics might reflect a risk of atherosclerosis and stenosis [8,9] in the aorta of Fontan. The negative correlation of axial WSS in DAo and EF delineated that higher axial WSS in DAo in patients might accompany with the deteriorated cardiac function. In conclusion, the cardiac dysfunction presented adverse interaction with aortic wall characteristics in patients with Fontan, especially in DAo, and might lead to higher risk of atherosclerosis and stenosis.Acknowledgements
No acknowledgement found.References
1. d'Udekem Y, et al. The Fontan procedure: contemporary techniques have improved long-term outcomes. Circulation. 2007 Sep 11;116(11 Suppl):I157-64.
2. Khairy P, et al. Univentricular heart. Circulation. 2007 Feb 13;115(6):800-12.
3. Khairy P, et al. Long-term survival, modes of death, and predictors of mortality in patients with Fontan surgery. Circulation. 2008 Jan 1;117(1):85-92.
4. Reneman, et al. Wall shear stress–an important determinant of endothelial cell function and structure–in the arterial system in vivo. J Vasc Res. 2006;43(3):251-69.
5. Ito S, et al. Left Ventricular Contractility and Wall Stress in Patients With Aortic Stenosis With Preserved or Reduced Ejection Fraction. JACC Cardiovasc Imaging. 2020 Feb;13(2 Pt 1):357-369.
6. Sotelo J, et al. 3D Quantification of Wall Shear Stress and Oscillatory Shear Index Using a Finite-Element Method in 3D CINE PC-MRI Data of the Thoracic Aorta. IEEE Trans Med Imaging. 2016 Jun;35(6):1475-87.
7. Shan Y, et al. Aortic shear stress in patients with bicuspid aortic valve with stenosis and insufficiency. J Thorac Cardiovasc Surg. 2017 Jun;153(6):1263-1272.e1.
8. Hashemi J, Patel B, Chatzizisis YS, Kassab GS. Study of Coronary Atherosclerosis Using Blood Residence Time. Front Physiol. 2021 May 3;12:625420.
9. Cho YI, Cho DJ, Rosenson RS. Endothelial shear stress and blood viscosity in peripheral arterial disease. Curr Atheroscler Rep. 2014 Apr;16(4):404.
Figures