4525
Predicting conversion from normal aging to mild cognitive impairment using 1H-MRS of posterior cingulate cortex1Lomonosov Moscow State University, Moscow, Russian Federation, 2Clinical and Research Institute of Emergency Pediatric Surgery and Trauma, Moscow, Russian Federation, 3Emanuel Institute of Biochemical Physics of RAS, Moscow, Russian Federation, 4Mental Health Research Center, Moscow, Russian Federation, 5Semenov Federal Research Center of Chemical Physics of the Russian Academy of Sciences., Moscow, Russian Federation
Synopsis
This study aims to identify possible biomarkers of minimal cognitive impairment (MCI) in the posterior cingulate cortex using MR spectroscopy. Absolute concentrations of metabolites such as Cr, NAA, Cho, Glx, Ins were determined in subjects with MCI and in subjects from the normal group. Our study revealed a statistically significant increase in the absolute concentration of choline-containing compounds, as well as the Cho/Cr value in PCC in the MCI group, what may indicate the destruction of cell membranes. The results expand our knowledge in understanding the causes of MCI and are promising for the study of Cho as biomarkers.
Introduction
This study examined the opportunity of proton magnetic resonance spectroscopy of posterior cingulate cortex (PCC) to predict the development of mild cognitive impairment (MCI) by identifying possible MCI biomarkers. MCI represents an intermediate stage between the cognitive decline of aging and the dementia characterized by a high rate of progression [1]. PCC is highly connected cerebral region with high metabolic activity and playing a central role in the default mode network of the brain [2]. It has been demonstrated that structural and functional abnormalities in this area are associated with cognitive impairment in neurodegenerative disorders [3]. Therefore, possible changes in the concentration of metabolites may expand our knowledge about MCI.Methods
The measurements were carried out on a Philips Ingenia 3.0 T scanner, 40 subjects participated in the study:1) Norm (without cognitive impairment) - 20 people, including 17 women and 3 men,(mean age = 65,7± 8,0)
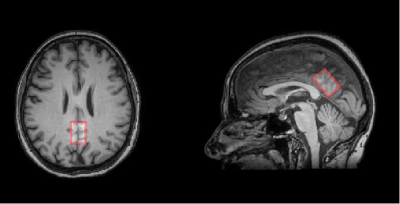
2) Patients with clinically diagnosed MCI - 20 people: 17 women and 3 men,(mean age = 69,3± 8,4). Spectroscopic voxel sized 20x20x30 mm was located in the PСC, its typical location is shown in Fig. 1
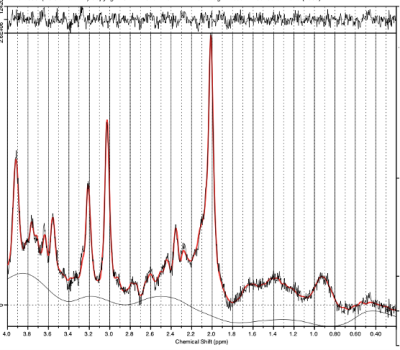
To acquire spectra, PRESS pulse sequence was used with the following parameters: TE = 35 ms,TR = 2000 ms, NSA = 80, the number of spectral points: 2048, BW = 2000 Hz. The reference spectrum of the unsuppressed water signal was also acquired. Spectral processing was carried out in LCModel, the intensities of the resonance lines of metabolites were determined, as well as the values of intensities normalized to Cr (Fig. 2) [4].
To calculate the absolute metabolite concentrations, the fact that the voxel contains various fractions such as gray matter (fGM), white matter (fWM), and cerebrospinal fluid (fCSF) was taken into account. FSL BET [5] was applied to remove tissues not belonging to cerebral structures such as fat, skin, bones, etc. After that, FSL FAST was used to determine value of fGM, fWM and fCSF [6]. To find the partial volumes, a mask was constructed in the Gannet program based on MATLAB. In such a way, voxels not belonging to the scanned area were excluded from the analysis [7].
Statistical processing was carried out in GraphPad Prism 8. To determine the significance of between-group differences, Brown-Forsythe and Welch ANOVA with multiple comparisons was used, P-values were false discovery rate-controlled. To determine the dependence of the absolute concentration of metabolites on age, correlation coefficients were found.
Results
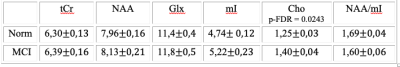
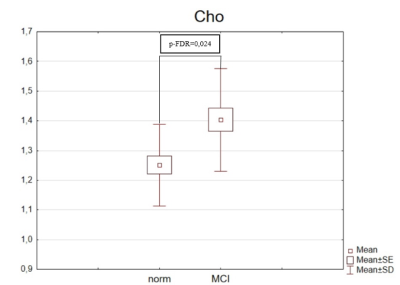
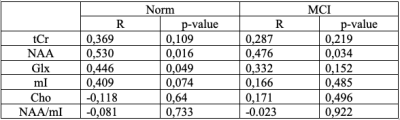
Average SNR of spectra of the normal subjects and of patients with MCI were 20 ± 4 and 19.6 ± 4.4, respectively. There is no statistical difference in age in the groups. The values of absolute concentrations of metabolites are presented in the table 1. The MCI group showed a statistically significant increase in the absolute concentration of Cho in PCC by 12%.The correlation between age and absolute concentration was weak for all metabolites in both groups, except for NAA in the normal group, where correlation was medium (see table 2):
Discussions
The literature data on the choline levels in MCI in contradictive. In the number of studies, e.g. [8] an increase in Cho/Cr in neurodegenerative diseases was found, while in [9] no changes have been detected. We found no literature data on the absolute metabolite concentrations alterations, so the reveled change in absolute [Cho] is reported for the first time.The absolute [Cho] does not significantly correlate with age of subjects from both groups, which is the evidence that the revealed increase of Cho is a characteristic of MCI and not normal aging.The main contribution to the intensity of the Cho signal in the brain spectra is made by glycerolphosphocholine and phosphocholine, which are a part of cell membranes [10]. Choline-containing compounds are important metabolites in the human brain, disruption of the ongoing biochemical processes with choline metabolites can lead to degradation of cell membranes, on which the normal functioning of the human brain depends.The study results are promising in terms of accepting the concentration of choline-containingcompounds as a biomarker of MCI. Further studies are required linking the magnitude ofmetabolic changes with the dynamics of the progression of cognitive impairment.Acknowledgements
No acknowledgement found.References
1. Roberts R, Knopman DS. Classification and epidemiology of MCI. Clin Geriatr Med. 2013 Nov;29(4):753-72. doi: 10.1016/j.cger.2013.07.003. PMID: 24094295; PMCID: PMC3821397
2. Leech R, Sharp DJ. The role of the posterior cingulate cortex in cognition and disease. Brain. 2014 Jan;137(Pt 1):12-32. doi: 10.1093/brain/awt162. Epub 2013 Jul 18. PMID: 23869106; PMCID: PMC3891440.
3. Buckner RL, Snyder AZ, Shannon BJ, LaRossa G, Sachs R, Fotenos AF, Sheline YI, Klunk WE, Mathis CA, Morris JC, Mintun MA. Molecular, structural, and functional characterization of Alzheimer's disease: evidence for a relationship between default activity, amyloid, and memory. J Neurosci. 2005 Aug 24;25(34):7709-17. doi: 10.1523/JNEUROSCI.2177-05.2005. PMID: 16120771; PMCID: PMC6725245.
4. Provencher SW. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn Reson Med. 1993 Dec;30(6):672-9. doi: 10.1002/mrm.1910300604. PMID: 8139448
5. S.M. Smith. Fast robust automated brain extraction. Human Brain Mapping, 17(3):143-155, November 2002.
6. Zhang, Y. and Brady, M. and Smith, S. Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. IEEE Trans Med Imag, 20(1):45-57, 2001, PMID: 11293691 DOI: 10.1109/42.906424
7. Richard A E Edden, Nicolaas A J Puts, Ashley D Harris, Peter B Barker, C John Evans. Gannet: A batch-processing tool for the quantitative analysis of gamma-aminobutyric acid–edited MR spectroscopy spectra.PMID: 25548816 PMCID: PMC4280680 DOI: 10.1002/jmri.24478
8. Kejal Kantarci. Proton MRS in mild cognitive impairment. PMID: 23526756 PMCID: PMC3609038 DOI: 10.1002/jmri.23800
9. Parnetti L, Tarducci R, Presciutti O, et al. Proton magnetic resonance spectroscopy can differentiate Alzheimer’s disease from normal aging. Mechanisms of Ageing &Development. 1997;97(1):9–14.
10. Membrane breakdown in acute and chronic neurodegeneration: focus on choline-containing phospholipids. J Klein
Figures