4523
Blood-Brain Barrier Leakage in Patients with Alzheimer’s Disease and Dementia with Lewy Bodies1Center for Biomedical Imaging Research, School of Medicine, Tsinghua University, Beijing, China, 2Department of Neurology, Beijing Tiantan Hospital, Capital Medical University, China National Clinical Research Center for Neurological Diseases, Beijing, China, 3Department of Neurology, Tianjin Huanhu Hospital, Tianjin, China, 4Department of Radiology, Tianjin Huanhu Hospital, Tianjin, China
Synopsis
Previous in vivo studies have shown that dysfunction of blood-brain barrier is highly associated with the process of Alzheimer’s disease (AD), but similar studies in dementia with Lewy bodies (DLB) have rarely been reported. In this study, for the first time, differences of BBB leakage among patients with AD, DLB and age-matched healthy control in different brain regions had been demonstrated, which may serve as one potential tool for diagnosis of different neurodegenerative diseases and contribute to our understanding of their pathophysiological basis.
Introduction
Alzheimer’s disease (AD) and dementia with Lewy bodies (DLB) are the most and second most common causes of dementia respectively1,2. Previous histologic evidence suggests that neurodegenerative diseases are highly associated with blood-brain barrier (BBB) leakage3. BBB leakage will cause several pathologic changes such as accumulation of neurotoxic proteins in central nervous system (CNS), which may lead to dementia finally4,5.In vivo quantitative evaluation of BBB leakage is hard to implement until the emergence of dynamic contrast-enhanced MRI (DCE-MRI), which provides accessibility to quantify the concentration of contrast agent permeating into brain parenchyma and calculate BBB leakage rates6. Recent in vivo studies have reported that BBB leakage rates in patients with AD is associated with cognitive decline 7-9. But studies in DLB and the differences of BBB leakage between AD and DLB have rarely been reported. Therefore, we aim to compare BBB leakage in patients with AD, DLB and healthy control subjects, and investigate its feasibility in distinguishing patients with different neurodegenerative diseases.Methods
Subjects: This prospective study was approved by the Ethics Committee, and all participants provided written informed consent. Patients with probable AD based on the National Institute on Aging and Alzheimer’s Association criteria10, patients with probable DLB based on the fourth consensus report of the DLB consortium11 and age-matched healthy elders were recruited. The exclusion criteria included physical disability, contraindications for MRI examination and major structural brain abnormalities. Demographic information of all subjects was also recorded.Imaging Protocol: Participants were scanned on a 3T MR imaging system (Magnetom Prisma, Siemens Healthcare, Erlangen, Germany) with a 64-channel head coil. For DCE-MR imaging, precontrast T1 mapping was performed using a 3D variable flip angle sequence. B1 map was also performed to correct B1 inhomogeneity. DCE images were acquired using a 3D T1-weighted spoiled gradient-echo sequence: TR/TE = 5.2/1.8 ms; FOV = 230 × 187 mm2; matrix size = 192 × 156; slice thickness = 3mm; number of slices = 56; imaging time = 30 × 13.58s. Coincident with the sixth dynamic scan, a bolus of 0.1 mmol/kg of Gd-DTPA (Magnevist; Bayer HealthCare Pharmaceuticals, Wayne, New Jersey) was injected intravenously at a rate of 2 mL/s, followed by a 12-mL saline flush at the same rate.
MR Imaging Analysis: Bilateral hippocampus, four brain lobes, precuneus and cingulate were regarded as regions of interest (ROI) in this study for their importance in cognitive function12. ROI were manually segmented on precontrast DCE images by two experienced radiologists who were blinded to the result of BBB leakage. Pharmacokinetics of contrast agent were analyzed using the Patlak model13 :
$$C(t)=K_{trans} \int_{0}^{t}C_p (τ)dτ+V_p C_p (t) $$
where $$$C(t)$$$ is the contrast agent concentration in the chosen tissue, $$$K_{trans} $$$represents the transfer rate of contrast agent from the intravascular space to the extravascular space, $$$C_p (t)$$$ is the vascular input function which was extracted from the superior sagittal sinus14, and $$$V_p$$$ represents the fractional plasma volume. The kinetic model was fitted using the least-squares method. ROI segmentation and post-processing analysis were both performed on MATLAB software (MathWorks, Natick, Massachusetts, USA).
Statistical Analysis: Continuous variables and categorical variables of the three groups were compared using one-way analysis of variance (ANOVA) and chi-square test respectively. In all subjects, bilateral Ktrans in different ROI was also compared using two-tailed independent Student’s t test. p<0.05 was considered as statistically significant and all statistical analyses were performed using R (UoA, Auckland, New Zealand).
Results
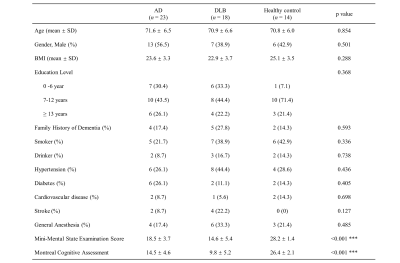
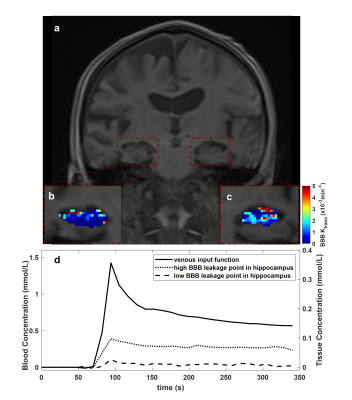
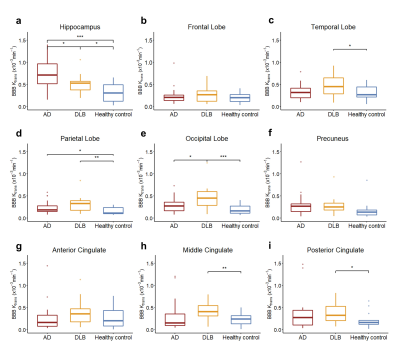
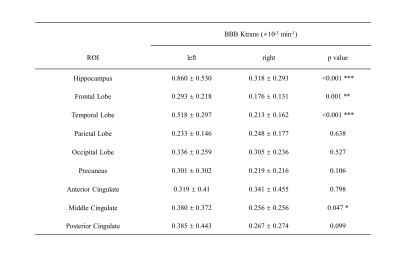
Between March 2021 and November 2021, 23 patients with AD, 18 patients with DLB and 14 age-matched healthy control were recruited in this study. No significant difference was found in demographic information except for Mini-Mental State Examination Score (MMSE) and Montreal Cognitive Assessment (MoCA) (Table 1). Precontrast DCE image, $$$K_{trans} $$$ map of bilateral hippocampus, blood concentration and tissue concentration of a patient with DLB (78-year-old man) are shown in Figure 1. A progressive significant increase of $$$K_{trans} $$$ was found in DLB group compared to healthy control group and AD group compared to DLB group in hippocampus (Figure 2). While in other ROI, mean $$$K_{trans} $$$ in DLB group was higher than that in AD group, but the difference is statistically significant only in occipital lobe. When compared to healthy control, DLB group shows significantly higher $$$K_{trans} $$$ in most chosen brain regions. Ktrans in left ROI was higher than that in corresponding ROI on the right, especially in hippocampus, frontal lobe, temporal lobe and middle cingulate (Table 2).Discussion and Conclusion
To the best of our knowledge, our study firstly reported the BBB leakage among patients with AD, DLB and healthy control. We found that Ktrans significantly differs between DLB and AD, DLB and healthy control in the hippocampus and occipital lobe. The observed difference of BBB leakage in different brain regions may contribute our understating of the pathophysiological mechanisms in DLB and AD and may serve as a supplementary marker. Studies are needed to validate the possible diagnostic value of $$$K_{trans} $$$ in a larger cohort. We also found that the BBB leakage in left brain regions is higher than that in right brain regions, which possibly relate to the structural and functional differences between the left and right brain regions.Acknowledgements
None.References
1. Blennow K, de Leon MJ, Zetterberg H. Alzheimer's disease. Lancet. 2006 Jul 29;368(9533):387-403.
2. Sanford AM. Lewy Body Dementia. Clin Geriatr Med. 2018 Nov;34(4):603-615.
3. Benarroch EE. Neurovascular unit dysfunction: a vascular component of Alzheimer’s disease? Neurology. 2007 May 15;68(20):1730-2.
4. Abbott NJ, Romero IA. Transporting therapeutics across the blood-brain barrier. Mol Med Today. 1996 Mar;2(3):106-13.
5. Sweeney MD, Zhao Z, Montagne A, Nelson AR, Zlokovic BV. Blood-Brain Barrier: From Physiology to Disease and Back. Physiol Rev. 2019 Jan 1;99(1):21-78.
6. Kumar S, Singh P, Vyas S, et al. Assessment of Blood-Brain Barrier Integrity in Tuberculous Meningitis Using Dynamic Contrast-Enhanced MR Perfusion. Indian J Radiol Imaging. 2021 Jan;31(1):30-36.
7. Montagne A, Barnes SR, Sweeney MD, et al. Blood-brain barrier breakdown in the aging human hippocampus. Neuron. 2015 Jan 21;85(2):296-302.
8. van de Haar HJ, Burgmans S, Jansen JF, van Osch MJ, van Buchem MA, Muller M, Hofman PA, Verhey FR, Backes WH. Blood-Brain Barrier Leakage in Patients with Early Alzheimer’s disease. Radiology. 2016 Nov;281(2):527-535.
9. Sweeney MD, Sagare AP, Zlokovic BV. Blood-brain barrier breakdown in Alzheimer’s disease and other neurodegenerative disorders. Nat Rev Neurol. 2018 Mar;14(3):133-150.
10. McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011 May;7(3):263-9.
11. McKeith IG, Boeve BF, Dickson DW, et al. Diagnosis and management of dementia with Lewy bodies: Fourth consensus report of the DLB Consortium. Neurology. 2017 Jul 4;89(1):88-100.
12. Poulose SM, Miller MG, Scott T, et al. Nutritional Factors Affecting Adult Neurogenesis and Cognitive Function. Adv Nutr. 2017 Nov 15;8(6):804-811.
13. Buckley DL, Shurrab AE, Cheung CM, Jones AP, Mamtora H, Kalra PA. Measurement of single kidney function using dynamic contrast-enhanced MRI: comparison of two models in human subjects. J Magn Reson Imaging. 2006 Nov;24(5):1117-23.
14. Taheri S, Gasparovic C, Shah NJ, et al. Quantitative measurement of blood-brain barrier permeability in human using dynamic contrast-enhanced MRI with fast T1 mapping. Magn Reson Med. 2011 Apr;65(4):1036-42.
Figures