4522
Selective Disruption of the Motor Connectome in Non-Familial Amyotrophic Lateral Sclerosis1Brain and Mind Centre; The University of Sydney, Sydney, Australia, 2Sydney Neuroimaging and Analysis Centre, Sydney, Australia, 3Department of Neurology, Royal Prince Alfred Hospital, Sydney, Australia
Synopsis
Amyotrophic lateral sclerosis (ALS) is a rapidly progressing neurodegenerative disorder with a widespread cortical disease signature. ALS patients demonstrate global network alterations in the white matter connectome in the early stage of disease. Selective disruption of cortical motor associated nodes with the thalamus and contralateral motor cortices is present at disease onset. Disease duration is associated with reduced structural interhemispheric cortical motor connectivity. Focal motor abnormalities present in the white matter connectome may be a sensitive marker in the earliest stages of ALS prior to functional decline.
Introduction
Amyotrophic lateral sclerosis (ALS) is a rapidly progressing neurodegenerative disorder characterized by functional motor decline. Presentation of clinical features at disease onset is heterogenous, including site of initial symptom onset and presence of extra-motor features (i.e., cognitive, behavioral, psychiatric)1. ALS shares a clinical, pathological, and genetic overlap with frontotemporal dementia, with the two conditions suggested to lie on opposing ends of a clinical continuum1, 2. Consequently, the neuroimaging signature of disease propagation in ALS is widespread and not limited to the motor system3. Notably, previous hypothesis driven studies by our group have demonstrated that degradation of frontal white matter connections, passing through the thalamus and corpus callosum, are significantly associated with rate of functional decline in ALS4, 5. The current study extends on these findings by examining the integrity of the structural connectome of white matter fibers throughout the brain, as a whole, in a clinically well-defined cohort of non-familial ALS patients using state-of-the-art multi-shell diffusion acquisition and fiber reconstruction.Methods
Thirty-one sporadic ALS patients and 16 age-education matched healthy control participants (p values > 0.4) were prospectively recruited from the Sydney Forefront Motor Neuron Disease Clinic, in accordance with ethical approval. All patients underwent comprehensive clinical examination by experienced neurologists (CM;WH;MK) and an MRI scan (3T GE MR750; 32 Channel Head Coil) on the same day. T1: MPRAGE, TR=6.2ms, TE=2.3ms, flip angle=12°, 1mm isotropic; DWI: 140 directions, b-values=0/700/1000/2800s/mm2 (8/25/40/75 volumes, respectively), 2mm isotropic. Anatomical T1 images were processed using the standard recon-all pipeline in FastSurfer6 to generate a whole-brain parcellation image (84 nodes, Desikan-Killiany atlas) and a 5-tissue-type (5TT) image (HSVS algorithm). Each participant’s whole-brain parcellation and 5TT images were co-registered to their mean b0 diffusion image. Diffusion processing was conducted using MRtrix3, and included standard DWI pre-processing (denoising, un-ringing, motion and distortion correction, and bias field correction), followed by estimation of response functions (dhollander algorithm) and constrained spherical deconvolution to estimate the fibre orientation distribution in each voxel, and subsequent normalisation of the fibre orientation distributions7. A whole-brain tractogram (10 million streamlines) was generated using anatomically-constrained tractography (iFOD2 algorithm), and filtered using SIFT28. SIFT2 normalised streamline weights were used to create a structural connectome corresponding to the whole-brain parcellation image. The following graph theory metrics were derived from each participant’s structural connectome using the Brain Connectivity Toolbox9: global efficiency, characteristic path length, local efficiency, betweenness centrality, modularity, strength, network degree, clustering coefficient, transitivity and density. Statistical comparisons were performed using SPSS V28. Group-wise differences in demographics variables and connectivity measures were assessed using independent samples t-tests and ANOVA with post-hoc comparisons, Bonferroni corrected. P-values < 0.05 were considered significant.Results
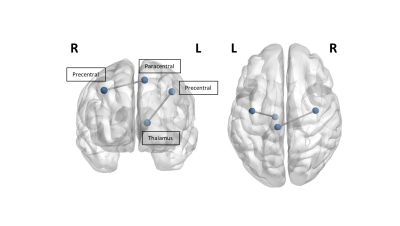
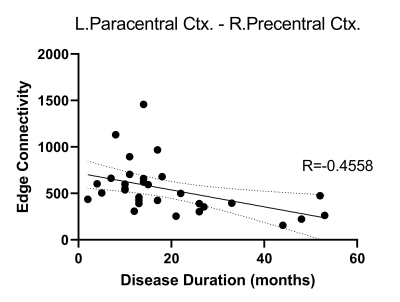
ALS patients demonstrated significant global network alterations, including reduced global efficiency (p=0.007), path length (p=0.012), local efficiency (p=0.01), strength coefficient (0.012), clustering coefficient (p=0.016), and transitivity (p=0.014), relative to healthy controls. A selective reduction in the connectivity strength of the left precentral cortex and left thalamus (p=0.032), as well as the left paracentral cortex and right precentral cortex (p=0.01), was observed in ALS patients (Fig. 1). Reduced edge connectivity between the left paracentral cortex and right precentral cortex was significantly associated with increased disease duration in ALS (Fig. 2; p=0.01). Post-hoc comparisons indicated a dissociative pattern of global network alterations between ALS patients with bulbar or limb site of initial symptom onset and healthy controls. Limb onset patients demonstrated reduced global efficiency (p=0.013), path length (p=0.05), and strength coefficient (p=0.05). In contrast, bulbar onset patients demonstrated reduced local efficiency (p=0.049), clustering (p=0.044), and transitivity (p=0.04).Discussion & Conclusions
Selective disruption affecting motor associated nodes of the white matter connectome is present at disease onset affecting connectivity with the left thalamus and contralateral motor cortex, consistent with our previous findings identifying the thalamus and corpus callosum as key disease structures associated with disease pathogenesis in ALS4, 5. Increased disease duration was associated with reduced interhemispheric motor connectivity, supporting existing neurophysiological evidence of disruption to transcallosal communication in the earliest stages of ALS10. Focal motor abnormalities present in the white matter connectome may be a sensitive marker in the earliest stages of ALS prior to functional decline.Acknowledgements
The authors thank all study participants and their carers for their efforts and enthusiasm.References
1. Kiernan MC, Vucic S, Cheah BC, et al. Amyotrophic lateral sclerosis. Lancet 2011;377:942-955.
2. Lillo P, Mioshi E, Burrell JR, Kiernan MC, Hodges JR, Hornberger M. Grey and white matter changes across the amyotrophic lateral sclerosis-frontotemporal dementia continuum. PLoS One 2012;7:e43993.
3. Westeneng HJ, Walhout R, Straathof M, et al. Widespread structural brain involvement in ALS is not limited to the C9orf72 repeat expansion. J Neurol Neurosurg Psychiatry 2016;87:1354-1360.
4. Tu S, Wang C, Menke RAL, et al. Regional callosal integrity and bilaterality of limb weakness in amyotrophic lateral sclerosis. Amyotroph Lateral Scler Frontotemporal Degener 2020;21:396-402.
5. Tu S, Menke RAL, Talbot K, Kiernan MC, Turner MR. Regional thalamic MRI as a marker of widespread cortical pathology and progressive frontotemporal involvement in amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry 2018;89:1250-1258.
6. Henschel L, Conjeti S, Estrada S, Diers K, Fischl B, Reuter M. FastSurfer - A fast and accurate deep learning based neuroimaging pipeline. Neuroimage 2020;219:117012.
7. Jeurissen B, Tournier JD, Dhollander T, Connelly A, Sijbers J. Multi-tissue constrained spherical deconvolution for improved analysis of multi-shell diffusion MRI data. Neuroimage 2014;103:411-426.
8. Smith RE, Tournier J-D, Calamante F, Connelly A. SIFT2: Enabling dense quantitative assessment of brain white matter connectivity using streamlines tractography. NeuroImage 2015;119:338-351.
9. Rubinov M, Sporns O. Complex network measures of brain connectivity: uses and interpretations. Neuroimage 2010;52:1059-1069.
10. Wittstock M, Wolters A, Benecke R. Transcallosal inhibition in amyotrophic lateral sclerosis. Clin Neurophysiol 2007;118:301-307.
Figures