4521
Hippocampal glutathione-glutamate couplings predict cognitive impairment in patients with relapsing-remitting multiple sclerosis1Department of Radiology, Shandong Provincial Hospital, Cheeloo College of Medicine, Shandong University, Jinan, China, 2Westa College, Southwest University, Chongqing, China, 3Philips Healthcare, Shanghai, China, 4Vanderbilt University Institute of Imaging Science, Nashville, TN, United States
Synopsis
Cognitive impairment is a common symptom of MS. GSH and Glu, keeping a homeostasis in the stable stage, were considered as key players in oxidative stress defending and synaptic plasticity, respectively. We aim to explore the changes of GSH and Glu levels and GSH-Glu couplings in RRMS and their association with cognitive impairment. Our findings indicate that oxidative stress and glutamatergic dysfunction may contribute to cognitive impairment of MS in a regional specificity manner. Hippocampal GSH-Glu decoupling may offer a crucial noninvasive measure of early cognitive impairment and provide a new strategy for the treatment of MS patients.
Purpose
Cognitive impairment is a common symptom of multiple sclerosis (MS) 1. Glutathione (GSH) and glutamate (Glu), keeping a homeostasis in the stable stage 2, were considered as key players in oxidative stress defending and synaptic plasticity, respectively. To explore the changes of GSH and Glu levels and GSH-Glu couplings in relapsing-remitting MS (RRMS) and their association with cognitive impairment.Methods
41 RRMS patients and 46 healthy controls (HCs) underwent magnetic resonance spectroscopy on a 3.0 T scanner to measure GSH and Glu levels in the posterior cingulate cortex, medial prefrontal cortex and left hippocampus, and underwent neuropsychological tests to assess cognitive function. Two-tailed t-test was used to estimate between-group differences in levels of metabolite. Then, the relationship between Glu and GSH in MS patients and HCs were analyzed using Pearson correlation coefficients. Hierarchical multiple linear regressions were used to evaluate the extent to which metabolite levels predicted performances on cognitive function.Results
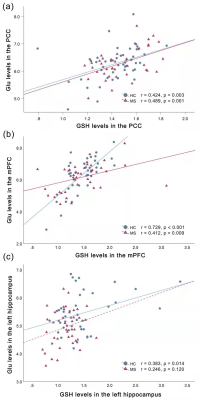
Compared to HCs, MS patients showed significantly lower hippocampal GSH (p = 0.001) and Glu (p = 0.003) levels, which were found to be significant predictors of worse visuospatial memory and verbal memory, respectively (ΔR2 = 0.250, p < 0.001; ΔR2 = 0.132, p = 0.006). Moreover, the absence of hippocampal GSH-Glu correlations were found in MS (r = 0.246, p = 0.120), and Glu/GSH ratios were better predictors of cognitive impairment than GSH levels.Discussion
GSH modulates neuroprotection from oxidative stress and is consumed in this course of antioxidants and detoxification3. Decreased Glu levels was found to occur simultaneously with demyelination in cuprizone mouse model of MS 4, indicating the dysfunctional glutamatergic system may be attributed to demyelination related to MS. We think the altered hippocampal GSH and Glu levels could reflect the oxidative stress and glutamatergic abnormality in MS patients. The involvement of the hippocampus in learning and memory is largely dependent on the glutamate-mediated signaling pathways in the hippocampus subfields 5. Moreover, hippocampal neurons need large amounts of GSH to maintain cognitive function 6. Abnormalities of GSH and Glu in the hippocampus may be predictive biological markers to detect the early changes of cognitive performance in patients with RRMS. Glu acts as a precursor for the synthesis of GSH, while GSH serve as a reserve pool for Glu neurotransmitter 2. As a result, Glu and GSH levels keep a homeostasis in the stable stage. Due to insufficient GSH synthesis, GSH-Glu couplings may be disrupted in MS patients, resulting in reduced GSH-Glu covariance. This dysregulation may underlie cognitive impairment in MS.Conclusion
Our findings indicate that oxidative stress and glutamatergic systems abnormality may contribute to cognitive impairment of MS in a regional specificity manner. Hippocampal GSH-Glu decoupling may offer a crucial noninvasive measure of early cognitive impairment and provide a new strategy for the treatment of patients with MS.Acknowledgements
This work was supported by the National Natural Science Foundation of China for Young Scholars (No. 81601479), Taishan Scholars Project (No. tsqn201812147), Jinan Science and Technology Development Program of China (Nos. 201907097 and 202019098), Shandong Provincial Medical and Healthy Technology Development Program of China (No. 202009010449), and Academic Promotion Programme of Shandong First Medical University (No. 2019QL023).References
1. Chiaravalloti ND, DeLuca J. Cognitive impairment in multiple sclerosis. The Lancet Neurology 2008;7:1139-1151.
2. Sedlak TW, Paul BD, Parker GM, et al. The glutathione cycle shapes synaptic glutamate activity. Proceedings of the National Academy of Sciences 2019.
3. Rae CD, Williams SR. Glutathione in the human brain: Review of its roles and measurement by magnetic resonance spectroscopy. Anal Biochem 2017;529:127-143.
4. Orije J, Kara F, Guglielmetti C, et al. Longitudinal monitoring of metabolic alterations in cuprizone mouse model of multiple sclerosis using 1H-magnetic resonance spectroscopy. Neuroimage 2015;114:128-135.
5. Tamminga CA, Southcott S, Sacco C, Wagner AD, Ghose S. Glutamate dysfunction in hippocampus: relevance of dentate gyrus and CA3 signaling. Schizophrenia bulletin 2012;38:927-935.
6. Fernandez-Fernandez S, Bobo-Jimenez V, Requejo-Aguilar R, et al. Hippocampal neurons require a large pool of glutathione to sustain dendrite integrity and cognitive function. Redox Biol 2018;19:52-61.
Figures
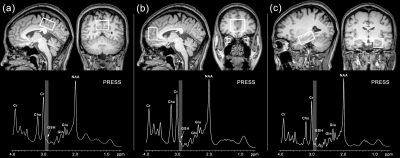
Figure 1 Positions of VOIs in PCC(a), mPFC (b), and left hippocampus(c) and corresponding Glu and GSH (thin white arrow) spectra obtained by a PRESS sequence. Cho: choline; Cr: creatine; Glu: glutamate; Gln: glutamine; GSH: glutathione; NAA: N-acetylaspartate; VOI: voxel of interest; PCC: posterior cingulate cortex; mPFC: medial prefrontal cortex.
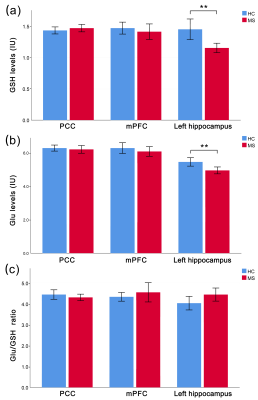
Figure 2. Comparisons of brain GSH levels between MS patients and HCs. Significantly lower GSH (p = 0.001) and Glu levels (p = 0.003) in the left hippocampus were shown in patients with MS, compared with HCs. ** indicate statistically significance levels of p < 0.01. No significant differences were observed in GSH or Glu levels in the mPFC and PCC between groups. MS: multiple sclerosis; HCs: healthy controls; Glu: glutamate; GSH: glutathione; PCC: posterior cingulate cortex; mPFC: medial prefrontal cortex.