4520
Gender-related differences in static and dynamic functional connectivity in multiple sclerosis: a multicenter study1Department of Radiology, The First Affiliated Hospital of Nanchang University, Nanchang, China, 2Department of Radiology, Beijing Tiantan Hospital, Beijing, China, 3MR Research, GE Healthcare, Beijing, China
Synopsis
The study investigated gender-related differences in patients with multiple sclerosis by assessing static and dynamic functional connectivity within large scale functional networks. Our results observed that MS could eliminate the effect of gender on static and dynamic functional connectivity in healthy controls, and affect static connectivity in male patients and dynamic connectivty in female patients. Correlation analyses suggested these characteristics of static and dynamic connectivity were related to disability and gray matter volume. Thus, our findings underline the importance of gender in founctional impairment and reorganization in MS
Introduction
Multiple sclerosis (MS) is a serious, progressive, and disabling disease, characterized by inflammatory demyelination in the central nervous system. The gender effects have been observed in patients with MS and affect the prevalence1, course2, disability3 and responses to therapy4. Recently, large cohort neuroimaging studies showed that MS males seem to have a higher level of brain damage compared to females5,6. These findings supported the idea that gender-related effects may affect brain structure in MS patients. However, it is not yet clear whether gender-related factors affect functional network connectivity in MS. Thus, our study aims to investigate gender-related differences in static and dynamic connectivity within large scale functional networks in MS, and the associations with structural and clinical measures.Materials and methods
A total of 208 patients with MS (135 females / 73 males) and 228 healthy controls (HC; 123 females / 105 males) were recruited from 7 Chinese centers for this retrospective study. All subjects underwent 3.0T scanner, and obtained high resolution 3D T1 weight, T2 weight, fluid-attenuated inversion recovery, and functional MR images. And physical disability of MS patients was measured by using the expanded disability status scale (EDSS). To define resting state networks, spatial independent component analysis was implemented using the Group ICA Of fMRI Toolbox, then obtained 20 components and 7 networks (Figure 1). To access the intra- and inter-network connectivity, spatial map intensity and functional network connectivity were analyzed. Moreover, the sliding window and k-means clustering methods were used to evaluate the connectivity pattern and variability of dynamic functional network connectivity (dFNC). Additionally, structural measurements were calculated, including T2 lesion load, total intracranial volume, white matter volume (WMV) and gray matter volume (GMV). Then, one-way ANOVA tests were used to assess the difference of static and dynamic connectivity at the group level (Healthy females vs. Healthy males, MS females vs. MS males, MS females vs. Healthy females, MS males vs. Healthy males; FDR corrected, P <0.05). Finally, partial correlations were used to analyze the associations between functional networks connectivity and structural, or clinical characteristics (P < 0.05).Results
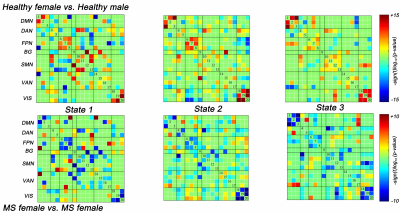
Demographic data and clinical characteristics are shown in Table 1 (Figure 2). In patients with MS, male patients seem to show younger ages (35.42±10.98 years), longer disease duration (median: 24 months), and higher disability (median EDSS: 2.5). Statistical analyses revealed that there is no significant difference in age, disease duration and EDSS score between female and male patients (P > 0.10). Moreover, MS males have more lesion load (P = 0.041, uncorrected) compared to MS females.In functional network analyses, widespread differences within intra- and inter-network static functional connectivity were observed between healthy males and females: stronger connectivity in females than males (Figure 3A, 3B). But these gender-related differences were significantly eliminated in MS patients. When compared to HC, the differences of static connectivity were observed in the male patients, not in female patients (Figure 3C, 3D), particularly decreased connectivity within ventral attention network, and between sensorimotor network and visual network (FDR correlated, P < 0.05).
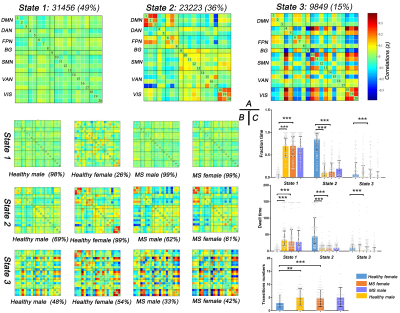
After cluster analysis, three dFNC states were identified for all participants: State 1 (weakly connected state; 49%), State 2 (middle-high connected state; 36%) and State 3 (high connected state; 15%) (Figure 4A). Healthy females preferred spending more time in middle-high connected state with fewer transitions, while healthy males and MS patients tended to stay in weakly connected state with frequent transitions (Figure 4C). Group-level analyses suggested gender-related dFNC differences in HC, which disappeared in MS. Most importantly, compared with healthy females, MS females showed more decreased connectivity in all states (Figure 5). While no dynamic significance between healthy and MS males. The dFNC results were corrected by FDR (P < 0.05).
Correlation analyses suggested functional connectivity within frontoparietal network (r = -0.251, P = 0.044) and between frontoparietal network and sensorimotor network (r = -0.311, P = 0.012) were negatively correlated with GMV in MS males. In MS females, EDSS scores (r = 0.216, P = 0.015) and GMV (r = -0.186, P = 0.037) were correlated with dell time in State 1, GMV (r = -0.291, P = 0.001) and WMV (r = -0.252, P = 0.004) were observed to be negatively related to the numbers of transition.
Discussion
Our results revealed that MS could eliminate the gender-related effect in healthy controls, and only male patients showed decreased functional connectivity and higher lesion load compared to HC. Our findings confirmed and further supported the idea that male patients have a higher degree of brain damage from functional evidence6,7. Contrary to static observations, decreased dFNC was displayed only in female patients. In addition, MS females tended to stay in weakly connected state, and the dell time is related to disability and GMV, which indicated that the dFNC damage occurred accompanying structural damage and clinical disability in MS females. Finally, MS females have higher number of transitions, suggesting that MS may impair the stability of dFNC patterns in females.Conclusion
Our results demonstrated that MS mainly affects static connectivity in males and dynamic connectivity in females, suggesting that gender-related effects may play an important role in functional impairment and reorganization of MS, and be related to clinical disability.Acknowledgements
We are grateful for support and discussions from our colleagues in other centers.References
1. Jobin C, Larochelle C, Parpal H, Coyle PK, Duquette P. Gender issues in multiple sclerosis: an update. Womens Health (Lond). 2010;6(6):797-820.
2. Bove RM, Healy B, Augustine A, et al. Effect of gender on late-onset multiple sclerosis. Mult Scler. 2012;18(10):1472-1479.
3. Ribbons KA, McElduff P, Boz C, et al. Male Sex Is Independently Associated with Faster Disability Accumulation in Relapse-Onset MS but Not in Primary Progressive MS. PLoS One. 2015;10(6):e0122686.
4. Magyari M, Koch-Henriksen N, Laursen B, Sorensen PS. Gender effects on treatment response to interferon-beta in multiple sclerosis. Acta Neurol Scand. 2014;130(6):374-379.
5. Pozzilli C, Tomassini V, Marinelli F, et al. 'Gender gap' in multiple sclerosis: magnetic resonance imaging evidence. Eur J Neurol. 2003;10(1):95-97.
6. Sanchis-Segura C, Cruz-Gomez AJ, Belenguer A, et al. Increased regional gray matter atrophy and enhanced functional connectivity in male multiple sclerosis patients. Neurosci Lett. 2016;630:154-157.
7. Schoonheim MM, Hulst HE, Landi D, et al. Gender-related differences in functional connectivity in multiple sclerosis. Mult Scler. 2012;18(2):164-173.
Figures
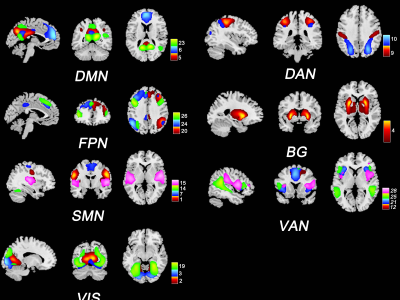
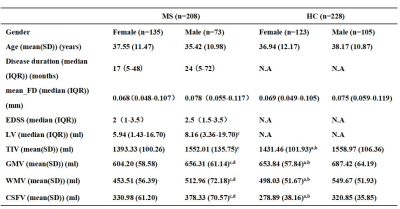
Figure 2 (Table 1): Demographic data and clinical characteristics. Abbreviation: MS, Multiple sclerosis; HC, healthy controls; IQR, interquartile range; mean_FD, mean framewise displacement; EDSS, expanded disability status scale; LV, lesion volume; TIV, total intracranial volume; GMV; gray matter volume; WMV, white matter volume; CSFV,cerebrospinal fluid volume; N.A, not applicable.
ameans P < 0.05 relative to healthy males
bmeans P < 0.05 relative to MS females
cmeans P < 0.05 relative to MS females
dmeans P < 0.05 relative to healthy males