4517
Demyelination and remyelination in cuprizone mouse model detected by CEST MRI at 3T1Department of Biomedical Engineering, City University of Hong Kong, Hong Kong, China, 2Department of Health Technology and Informatics, The Hong Kong Polytechnic University, Hong Kong, China, 3Russell H. Morgan Department of Radiology and Radiological Science, The Johns Hopkins University School of Medicine, Baltimore, MD, United States, 4City University of Hong Kong Shenzhen Research Institute, Shenzhen, China, 5Hong Kong Centre for Cerebro-Cardiovascular Health Engineering (COCHE), Hong Kong, China
Synopsis
Multiple sclerosis (MS) is a common demyelinating disease that heavily relies on differential diagnosis. Specific CEST contrast is known to be sensitive to alterations in proteins and lipids, the major components of myelin. This includes amide protons at 3.5 ppm and relayed nuclear Overhauser effect (rNOE) at -1.6~-3.5 ppm. Here, we study these CEST contrasts and their uniqueness towards myelin changes in a cuprizone model, which recapitulates remyelination and demyelination in MS. We observed substantial changes of rNOE and amide, during demyelination (P<0.05) and remyelination, indicating great potential of CEST MRI in monitoring myelin change and MS identification at 3T.
INTRODUCTION
Multiple sclerosis (MS) is the most common demyelinating disorder of the central nervous system, predominantly affecting young adults1,2. Remyelination has been introduced as one of the strategies for neuroprotection, nevertheless, the prevention of disability has yet to be achieved3. Sensitive imaging approach is desired to detect myelin loss and regeneration for early diagnosis and treatment. Chemical exchange saturation transfer (CEST) magnetic resonance imaging (MRI) is a non-invasive molecular imaging approach, which is capable of mapping endogenous molecules at low concentrations by detecting the exchangeable protons between solutes and bulk water4,5. Amide CEST6,7 and and relayed nuclear Overhauser effect (rNOE) CEST8,9 have been reported in detecting proteins and lipids, which are major components of myelin. Amide proton transfer-weighted imaging, which is typically analyzed using MTR asymmetry, contains the contributions from both amide and rNOE. It is reported for myelin detection10. Recently, we also demonstrated the identification of MS patients using rNOE11. Here, we used cuprizone model, a well-established mouse model with myelin pathology in corpus callosum (CC)2,12,13, to study CEST MRI (rNOE and amide) in detecting demyelination and remyelination in brain at 3T. Significant CEST signal changes within CC were observed and could indicate the corresponding changes in myelin, which were validated by immunohistochemistry.METHODS
Nineteen C57BL/6 mice (male, 8 weeks old) used in this study were randomly divided into control (CTL, n=9) and cuprizone (CPZ, n=10) groups. CTL mice were fed with control diet for the whole course, while CPZ mice were fed with diet containing 0.2% (w/w) cuprizone for 10 weeks (demyelination) followed by 4 weeks with control diet (remyelination), as shown in Fig. 1A. MRI scans were performed on a 3T Bruker BioSpec system (Bruker, Germany). The 2 mm-thick image slice containing CC was positioned on a collected sagittal image (Fig. 1C). CEST data was acquired using pulsed-CEST (VDMP) module followed by a RARE readout sequence14. The saturation parameters were as following11: saturation power=0.8 μT, pulsed width=40 ms, mixing time=60 ms and pulse number=30. Frequency offsets were set to -13~13 ppm for Z-spectra and 200 ppm for M0 image (normalization). Other parameters were set as following: TR=5000 ms, TE=3.69 ms, FOV=16×16 mm2, matrix=96×96, scan time=24 min. T1 maps of mouse brains were measured using RAREVTR sequence. CEST data was firstly corrected with B0 inhomogeneity. Then, rNOE (-3.5 ppm) and amide (3.5ppm) contrasts were extracted using Lorentzian-difference method9 and corrected by T1 map (AREX)15-20. Regions of brain and CC were drawn for analysis (Fig. 1D). Immunohistochemistry was performed and results at week 8 were shown here to validate MRI findings.RESULTS AND DISCUSSION
We summarized the experimental scheme and slice placement in Fig. 1. Mouse weight was recorded and showed no differences between groups during the study (Fig. 1B). In CC, both rNOE and amide CEST contrasts were comparable initially at week 0 between CTL and CPZ groups. A decrease in rNOE and amide CEST were observable in maps at -3.5 and at 3.5 ppm, respectively, at week 8 post cuprizone diet (Fig. 2A and 3A). rNOE was significantly lower in CPZ group when compared to CTL group (P<0.01, Fig. 2B). Similarly, amide CEST contrast was significantly lower in CPZ group (P<0.05, Fig. 3B). After week 10, cuprizone diet of CPZ group was replaced by control diet for remyelination. Interestingly, at week 14, rNOE contrast in CPZ group recovered to a similar level as that in CTL group (P>0.05, Fig. 2B), indicating both the cuprizone-induced demyelination and remyelination in CC can be sensitively detected by rNOE contrast. This slight increase in rNOE from week 8 to 14 in CPZ during remyelination, leading to a comparable level to CTL, was not observed in amide CEST. The amide contrast remained lower in CPZ than that in CTL at week 14 (Fig. 3B). For whole brain, a slight decrease at week 8 but a slight increase at week 14 in rNOE contrast (P>0.05) were observed (Fig. 2C), while amide contrast slightly decreased (P>0.05) during the course of our study (Fig. 3C). Immunohistochemistry was used to validate the MRI observations. An obviously lower fluorescent myelin signal of CC relative to cortex was found in CPZ than in CTL (0.9 vs. 1.2, Fig. 4), which supported the observations of CEST MRI at week 8.The distinctive observations at week 14, i.e. a slight increase in rNOE vs a decrease in amide CEST contrast could indicate that rNOE contrast has a high sensitivity towards lipid as myelin contains over 70% of lipids. Moreover, we also demonstrated that rNOE could sensitively detect demyelination in MS patients11.
CONCLUSION
Significant decrease in rNOE and amide CEST contrasts were observed in cuprizone-induced demyelination mouse model at week 8, as validated by immunohistochemistry. Interestingly, we observed a slight increase in rNOE contrast of CPZ, which has a similar contrast as CTL at week 14 during remyelination. This was not observed in amide CEST. These results indicate that both rNOE and amide contrasts can detect demyelination, while rNOE contrast showed a potential sensitivity in detecting remyelination. Overall, our findings indicated that CEST MRI is capable of monitoring demyelination/remyelination and has great potential in identification of myelin-related neuropathology, such as MS at 3T11.Acknowledgements
Authors are grateful to receive funding support from the Research Grants Council: 11102218; City University of Hong Kong: 7005210, 7005433, 9680247, 9667198 and 9609307; National Natural Science Foundation of China: 81871409; Health and Medical Research Fund: 05163736. Jianpan HUANG acknowledges funding from Research Grants Council: PDFS2122-1S01.
References
1. Thompson AJ, Baranzini SE, Geurts J, et al. Multiple sclerosis. Lancet 2018;391(10130):1622-1636.2. Zhan J, Mann T, Joost S, et al. The Cuprizone Model: Dos and Do Nots. Cells 2020;9(4):843.
3. Lubetzki C, Zalc B, Williams A, et al. Remyelination in multiple sclerosis: from basic science to clinical translation. Lancet Neurol 2020;19(8):678-688.
4. Ward KM, Aletras AH, Balaban RS. A new class of contrast agents for MRI based on proton chemical exchange dependent saturation transfer (CEST). J Magn Reson 2000;143(1):79-87.
5. van Zijl PC, Yadav NN. Chemical exchange saturation transfer (CEST): what is in a name and what isn't? Magn Reson Med 2011;65(4):927-948.
6. Zhou J, Payen JF, Wilson DA, et al. Using the amide proton signals of intracellular proteins and peptides to detect pH effects in MRI. Nat Med 2003;9(8):1085-1090.
7. Jones CK, Schlosser MJ, van Zijl PC, et al. Amide proton transfer imaging of human brain tumors at 3T. Magn Reson Med 2006;56(3):585-592.
8. van Zijl PC, Zhou J, Mori N, et al. Mechanism of magnetization transfer during on-resonance water saturation. A new approach to detect mobile proteins, peptides, and lipids. Magn Reson Med 2003;49(3):440-449.
9. Jones CK, Huang A, Xu J, et al. Nuclear Overhauser enhancement (NOE) imaging in the human brain at 7T. Neuroimage 2013;77:114-124.
10. Lee DW, Heo H, Woo DC, et al. Amide Proton Transfer-weighted 7-T MRI Contrast of Myelination after Cuprizone Administration. Radiology 2021;299(2):428-434.
11. Huang J, Xu J, Lai JHC, et al. Relayed nuclear Overhauser effect weighted (rNOEw) imaging identifies multiple sclerosis. Neuroimage 2021;32:102867.
12. Stidworthy MF, Genoud S, Suter U, et al. Quantifying the early stages of remyelination following cuprizone-induced demyelination. Brain Pathol 2003;13(3):329-339.
13. Skripuletz T, Gudi V, Hackstette D, et al. De- and remyelination in the CNS white and grey matter induced by cuprizone: the old, the new, and the unexpected. Histol Histopathol 2011;26(12):1585-1597.
14. Xu J, Yadav NN, Bar-Shir A, et al. Variable delay multi-pulse train for fast chemical exchange saturation transfer and relayed-nuclear overhauser enhancement MRI. Magn Reson Med 2014;71(5):1798-1812.
15. Zaiss M, Windschuh J, Paech D, et al. Relaxation-compensated CEST-MRI of the human brain at 7T: Unbiased insight into NOE and amide signal changes in human glioblastoma. Neuroimage 2015;112:180-188.
16. Rerich E, Zaiss M, Korzowski A, et al. Relaxation-compensated CEST-MRI at 7 T for mapping of creatine content and pH--preliminary application in human muscle tissue in vivo. NMR Biomed 2015;28(11):1402-1412.
17. Zaiss M, Windschuh J, Goerke S, et al. Downfield-NOE-suppressed amide-CEST-MRI at 7 Tesla provides a unique contrast in human glioblastoma. Magn Reson Med 2017;77(1):196-208.
18. Goerke S, Soehngen Y, Deshmane A, et al. Relaxation-compensated APT and rNOE CEST-MRI of human brain tumors at 3 T. Magn Reson Med 2019;82(2):622-632.
19. Huang J, Han X, Chen L, et al. Relayed nuclear Overhauser enhancement imaging with magnetization transfer contrast suppression at 3 T. Magn Reson Med 2021;85(1):254-267.
20. Zhang XY, Wang F, Li H, et al. Accuracy in the quantification of chemical exchange saturation transfer (CEST) and relayed nuclear Overhauser enhancement (rNOE) saturation transfer effects. NMR Biomed 2017;30(7):10.1002/nbm.3716.
Figures
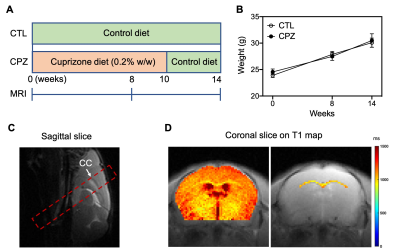
Fig. 1. (A) Experimental schemes of diet and MRI for control (CTL) and cuprizone (CPZ) groups. (B) Mouse weights of CTL and CPZ groups at different timepoints. (C) Slice selection (red frame) containing corpus callosum (CC) on sagittal image. (D) Regions of interest for whole brain and CC at coronal slice on T1 map.
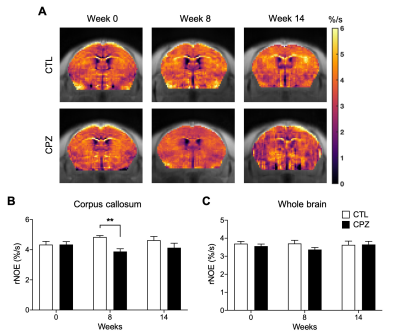
Fig. 2. (A) Representative rNOE AREX maps for mouse brains of CTL and CPZ. Comparison of rNOE AREX signal between CTL and CPZ for (B) corpus callosum and (C) whole brain. Data was presented as mean ± SEM and analyzed via two-way ANOVA.
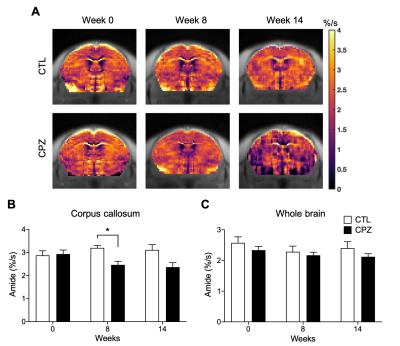
Fig. 3. (A) Representative amide AREX maps for mouse brains of CTL and CPZ. Comparison of amide AREX signal between CTL and CPZ for (B) corpus callosum and (C) whole brain. Data was presented as mean ± SEM and analyzed via two-way ANOVA.
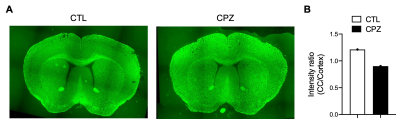
Fig. 4. (A) Fluorescent myelin stained histology for mouse slices of CTL and CPZ at week 8. (B) Comparison of intensity ratio between corpus callosum (CC) and cortex.