4515
Disentangling characteristics of subpial lesions in multiple sclerosis using multiparametric postmortem MRI1Neurology Clinic and Policlinic, Departments of Medicine, Clinical Research and Biomedical Engineering, University Hospital Basel and University of Basel, Basel, Switzerland, 2Translational Imaging in Neurology (ThINk) Basel, Department of Biomedical Engineering, University Hospital Basel and University of Basel, Basel, Switzerland, 3Research Center for Clinical Neuroimmunology and Neuroscience (RC2NB) Basel, University Hospital Basel and University of Basel, Basel, Switzerland, 4Institute of Neuropathology, University Medical Center Göttingen, Göttingen, Germany, 5Clinical Trial Unit, Department of Clinical Research, University Hospital Basel, University of Basel, Basel, Switzerland, 6Department of Cognitive Neurology, MR-Research in Neurosciences, University Medical Center Göttingen, Göttingen, Germany, 7Centre for Research in Neuroscience - Department of Clinical Neurosciences, Laboratoire de recherche en neuroimagerie (LREN) University Hospital and University of Lausanne, Lausanne, Switzerland, 8National Institute of Neurological Disorders and Stroke, Bethesda, MD, Bethesda, MD, United States
Synopsis
We have characterized the imaging correlates of subpial demyelination in the cerebral cortex of MS patients by exploiting multiparametric postmortem qMRI and histopathology. MTsat, qT1 and AD were the measures that best captured subpial lesions pathology. Additionally, we found that some subpial lesions exhibit a juxta-cortical rim of increased susceptibility and show lower MWF than the ones without rim.
Introduction
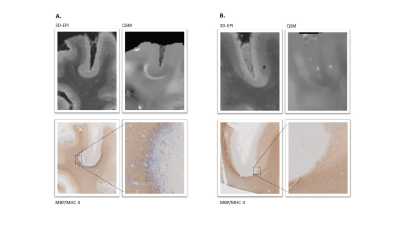
Histopathological1 and neuroimaging studies2 revealed the presence of extensive grey matter pathology in multiple sclerosis (MS) patients. The predominant form of cortical damage in MS is represented by subpial demyelination, which typically extends from the outer to the inner layers of the cortex3. Subpial lesions are specific to MS and predominant in the progressive phase of the disease4. Focal damage to the cerebral cortex is clinically relevant, because the cortical lesions load is associated with cognitive and physical disability and predicts disease progression5. Still, subpial lesions are heterogeneous and show different grades of inflammatory activity1; to date, little is known about their microstructural characteristics. In this study, we exploited postmortem MRI and histopathology of MS brains to investigate microstructural damage in subpial lesions by using multiparametric quantitative MRI. Further, we studied the pathological meaning of a juxta-cortical rim of increased susceptibility in a subgroup of subpial lesions (Figure 1).Methods
Two brains from two deceased MS patients (progressive and relapsing remitting, disease duration: 23 and 17 years) were imaged on a 3T whole-body MR system using a 20-channel head and neck coil and a dome-shaped brain container filled with perfluoropolyether. The brains had been fixed in 4% formalin within 24 hours from death and MRI was performed after 3 to 12 months. Images were acquired with the following spatially isotropic sequences, adapted to ex vivo conditions and capabilities: (i) a T1-weighted (TR=11.33ms, FA 15deg), proton density weighted (TR=25ms, FA 5deg), and MT prepared (TR=25ms, FA 5deg) 3D RF-spoiled gradient echo sequence of identical geometry (570μm isotropic) to allow MTsat map reconstruction6, (ii) fast T2prep sequence with spiral readout trajectory (1000μm isotropic, TEprep=[0, 7.5, 17.5, 67.5, 147.5, 307.5]ms, TRreadout=9.3ms) to assess myelin water fraction (MWF)7, (iii) MP2RAGE (670μm isotropic, TR=5s, TE=1.78ms, TI1=194ms and TI2=2500ms) to obtain quantitative T1 maps (qT1)8; (iv) segmented 3D-EPI (330μm isotropic, TR=65ms, TE=35ms, ETL=13, bandwidth 394Hz/Pixel) to enable quantitative susceptibility mapping (QSM)9; and (v) diffusion tensor imaging: for Brain #1: 1.5mm isotropic resolution, b-value=0/1650/2350/4650 s/mm2, TE=99.0ms, δ=31.9ms, Δ=45.9ms; Brain #2: 1.3mm isotropic resolution, b-value 1350/2650/4000 s/mm2, TE=80.0ms; δ=22.3ms; Δ=36.3ms to compute fractional anisotropy (FA), radial diffusivity (RD) and axial diffusivity (AD) maps after denoising10. In order to ease the registration of the MRI to the histology slices, we designed and 3D-printed an individualized cutting box based on the MRI for each brain, as reported in11. We performed histopathological analysis on 21 subpial lesions and 21 corresponding areas of normal appearing grey matter (NAGM). On the brain slices of the two brains, subpial lesions and a corresponding region of NAGM nearby were identified after staining with immunohistochemical double staining of histological slices of tissue blocks for Myelin Basic Protein (MBP, for myelin) and MHC II (for microglia/macrophages). Cortical lesions and NAGM were then identified on histology-matched 3D-EPI and 2D segmented manually on two dimensions using ITK-SNAP 3.6.012. Subpial lesions with or without a rim in the underlying WM were manually classified and then 3D segmented in the only brain showing this peculiar lesion type (brain #1). Statistical analyses were performed using Wilcoxon signed-rank test for the histology-MRI matched analysis of subpial lesions and Bonferroni correction was applied to account for multiple testing. Mann–Whitney test was used to explore the presence of microstructural differences between subpial lesions with and without a juxta-cortical rim.Results
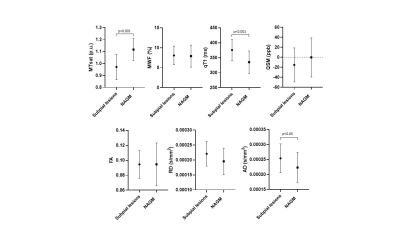
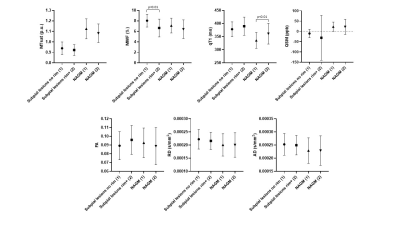
In subpial lesions (n = 21), MTsat was lower (p<0.001) and qT1/AD were higher (p<0.001 and p<0.05, respectively) compared to the adjacent NAGM (Figure 2). We identified a total of 50 subpial lesions in 3D-EPI images of brain #1. Of these, 20 showed a rim of increased susceptibility at the white matter/grey matter border. On histology, this juxta-cortical rim corresponded to microglia accumulation (Figure 1). MWF was higher in subpial lesions without rim compared to subpial lesions with rim (Figure 3).Discussion
Subpial lesions exhibited lower MTsat, higher qT1 and higher AD than NAGM. This might point to a different sensitivity to tissue damage in this lesion type (e.g. AD vs FA and RD), or to the higher spatial resolution of MTsat and qT1 compared to MWF. The observed increase in AD is in line with the results reported in previous studies13 and is probably due to a prevailing loss of parallel axons in the demyelinated cortex. The juxta-cortical rim of increased susceptibility exhibited by some subpial lesions corresponded to microglia/macrophages accumulation. Higher MWF in non-rim lesions may be due to a low intracellular (and extracellular) water content. This finding is compatible with lower intralesional cellularity, which might indicate a late-stage of lesion development (e.g. higher neuronal damage and lower astrocyte content). Future studies will investigate neuronal and glial density in subpial lesions with and without rim.Conclusion
We provided first evidence of the most sensitive quantitative MRI measures to study the microstructure of subpial lesions and identified a specific characteristic of some of these lesions (juxta-cortical rim), which possibly indicates early-stage cortical damage.Acknowledgements
No acknowledgement found.References
1. Lucchinetti CF, Popescu BFG, Bunyan RF, Moll NM, Roemer SF, Lassmann H, et al. Inflammatory cortical demyelination in early multiple sclerosis. N Engl J Med. 2011 Dec 8;365(23):2188–97.
2. Calabrese M, Rocca MA, Atzori M, Mattisi I, Favaretto A, Perini P, et al. A 3-year magnetic resonance imaging study of cortical lesions in relapse-onset multiple sclerosis. Ann Neurol. 2010 Mar;67(3):376–83.
3. Peterson JW, Bö L, Mörk S, Chang A, Trapp BD. Transected neurites, apoptotic neurons, and reduced inflammation in cortical multiple sclerosis lesions. Ann Neurol. 2001 Sep;50(3):389–400.
4. Junker A, Wozniak J, Voigt D, Scheidt U, Antel J, Wegner C, et al. Extensive subpial cortical demyelination is specific to multiple sclerosis. Brain Pathol Zurich Switz. 2020 May;30(3):641–52.
5. Harrison DM, Roy S, Oh J, Izbudak I, Pham D, Courtney S, et al. Association of Cortical Lesion Burden on 7-T Magnetic Resonance Imaging With Cognition and Disability in Multiple Sclerosis. JAMA Neurol. 2015 Sep;72(9):1004–12.
6. Tabelow K, Balteau E, Ashburner J, Callaghan MF, Draganski B, Helms G, et al. hMRI - A toolbox for quantitative MRI in neuroscience and clinical research. NeuroImage. 2019 Jul 1;194:191–210.
7. Nguyen TD, Deh K, Monohan E, Pandya S, Spincemaille P, Raj A, et al. Feasibility and reproducibility of whole brain myelin water mapping in 4 minutes using fast acquisition with spiral trajectory and adiabatic T2prep (FAST-T2) at 3T. Magn Reson Med. 2016 Aug;76(2):456–65.
8. Marques JP, Kober T, Krueger G, van der Zwaag W, Van de Moortele P-F, Gruetter R. MP2RAGE, a self bias-field corrected sequence for improved segmentation and T1-mapping at high field. NeuroImage. 2010 Jan 15;49(2):1271–81.
9. Liu C, Li W, Tong KA, Yeom KW, Kuzminski S. Susceptibility-weighted imaging and quantitative susceptibility mapping in the brain. J Magn Reson Imaging JMRI. 2015 Jul;42(1):23–41.
10. Veraart J, Novikov DS, Christiaens D, Ades-Aron B, Sijbers J, Fieremans E. Denoising of diffusion MRI using random matrix theory. NeuroImage. 2016 Nov 15;142:394–406.
11. Luciano NJ, Sati P, Nair G, Guy JR, Ha S-K, Absinta M, et al. Utilizing 3D Printing Technology to Merge MRI with Histology: A Protocol for Brain Sectioning. J Vis Exp JoVE. 2016 Dec 6;(118).
12. Yushkevich PA, Piven J, Hazlett HC, Smith RG, Ho S, Gee JC, et al. User-guided 3D active contour segmentation of anatomical structures: significantly improved efficiency and reliability. NeuroImage. 2006 Jul 1;31(3):1116–28.
13. Preziosa P, Kiljan S, Steenwijk MD, Meani A, van de Berg WDJ, Schenk GJ, et al. Axonal degeneration as substrate of fractional anisotropy abnormalities in multiple sclerosis cortex. Brain. 2019 Jul 1;142(7):1921–37.
Figures