4510
A multinuclear 4-channel 2H loop and 4-channel 1H microstrip array coil for human head MRS/MRI at 7T1Center for Magnetic Resonance Research (UMN), Minneapolis, MN, United States
Synopsis
A 4-channel 2H loop and 4-channel 1H microstrip head array coil with improved MRS/MRI sensitivity and imaging coverage for a human-head-like lightbulb phantom at 7T is reported. Each of the 4 2H coils is a 22cmx14cm loop, providing excellent penetration of 2H MRS signal. The 2H loops are decoupled by multiple techniques including inductive decoupling circuits and geometric overlapping, providing S12~-20dB between any pair of 2H loop coils. The majority of measured signal-to-noise ratio of the water signal originating from the natural abundance HDO content are above 100 for the voxel size of 1.7 cc.
Introduction:
Deuterium MRS imaging (DMRSI) is a promising tool with strongly increasing popularity for imaging cerebral glucose metabolism including the tricarboxylic acid cycle (TCA cycle)[1]. DMRSI offers higher sensitivity and less technique complexity than 13C MRS; it is also less invasive and more cost-effective than the clinically available, but ionizing-radiating imaging modality - fluorodeoxyglucose (FDG) positron emission tomography (PET). Through administration of deuterated glucose, the Warburg effect map (Lac/Glx) generated by DMRSI revealed high contrast between tumor and normal tissue[2,3]. An 8TxRx/2Rx 2H phased array at 9.4T[4] showed higher DMRS signal in the peripheral area and relative weak signal in the center of a head-size deuterated water phantom. As 2H coil B1 field wavelength is seven times bigger than that of proton in the same field strength, the majority of 2H B1 field is spatially distributed close to the conductor of the coil. This deteriorates the 2H B1 field penetration in the imaging phantom as compared to that of a 1H coil, and dominates the coupling between 2H array coils.In this study, we report a novel combination of 4 channel 2H loop coils and 4 channel 1H microstrip elements (i.e., 4-ch 2H TxRx/4-ch 1H TxRx head array coil). Four large 2H loops were overlapped with each other, providing an excellent 2H signal coverage and uniform distribution inside a head-size phantom.
Method:
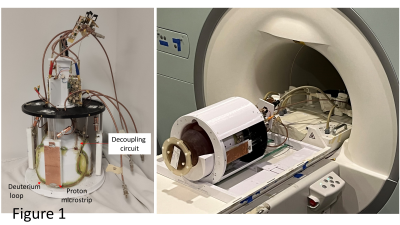
The four channel 2H loop coils (width x height: 22cmx14cm) are overlaid around a head helmet of size 23.5cmx19.5cm (Fig. 1). The non-adjacent 2H loops are decoupled using a resonant inductive decoupling circuit (RID) [5] elongated by coaxial cable at both ends, and the adjacent 2H loops are decoupled using the geometric overlapping technique. Four 1H microstrip elements (15 cm long) [6], each having a shorter physical length than that of a traditional 1H dipole at 7T, are positioned in the middle of the 2H loops. The 1H microstrip is mechanically easy to install, and shows less coupling with other electronic components (diodes, preamps, etc.).A head-size lightbulb-shaped phantom (17cm in length, and 17 cm in diameter) with similar loading as the human head was used for loading the array coil in all bench tests and imaging experiments. The four channel 2H coils were driven in a circularly polarized (CP) manner, with each of the 4 elements transmitting equal magnitudes with the appropriate phase shift, and each channel receives the 2H signal independently. A high-resolution chemical shift imaging (CSI) sequence was used to acquire DMRSI data: FOV 20x20x20 cm3, TR = 540 ms, matrix size = 17x17x13 (nominal size of cylindrical voxel = π0.6x0.6x1.5 cm3), TA = 8:54 min. For processing the 2H CSI spectra, a 10-Hz line broadening was applied, number of points for the left shift was 3, the zero-order phase (rp) for each 2H channel is shown in Table 1. The phase corrected CSI of four 2H channels were added together to generate the combined 2H CSI. The signal-to-noise ratio (SNR) for each CSI voxel was calculated as the resonance intensities of the combined CSI spectra divided by the noise level of the combined CSI spectra. The four 1H microstrip elements were driven in CP mode, and the combined images are co-registered with the 2H CSI images.
Results:
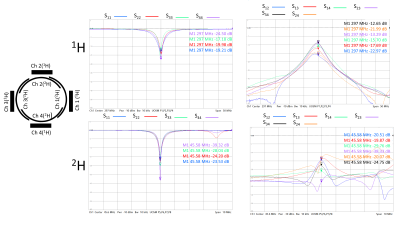
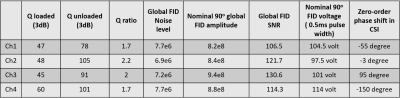
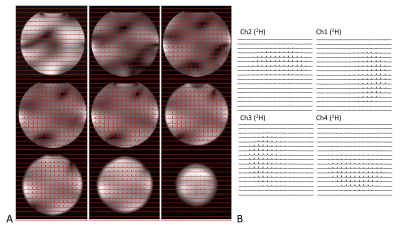
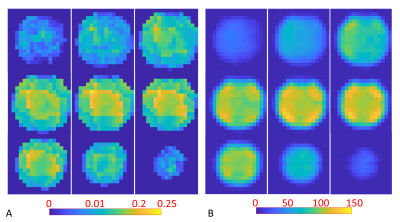
Fig. 1 shows the prototype of the 4-ch 2H TxRx/4-ch 1H TxRx head array coil and the set up for performing 1H MRI and 2H MRSI at 7T. The S parameters of the 1H microstrip and 2H loop coils are shown in Fig. 2, indicating excellent decoupling with the S12 of ~-20dB or better among all 2H loop coils.Fig. 3A shows good signal coverage of four 2H channels combined CSI overlay on co-registered proton images across a 13.8 cm slab (nine CSI slices, each with 1.54 cm thickness; axial orientation). Fig. 3B shows phase corrected individual CSI of four 2H channels in the same slice location at the center of Fig. 3A. Each 2H channel demonstrates consistent water signal distribution and spectral phases across the entire CSI after phase correction, indicating excellent decoupling between 2H coils. Table 1 summarizes the Q factors and global FID noise, amplitude and SNR of each 2H channel. The nominal 90-degree voltage for each channel was ~100 volts at 0.5 ms pulse length, and the zero-order phase shift (rp) for each channel was close to 90 degree apart. The four channels’ combined B1+ and SNR maps are shown in Fig. 4A and Fig. 4B, respectively. The majority of measured SNRs of the 2H signal originating from the natural abundance HDO content are above 100 for the nominal voxel size of 1.7cc (Fig. 4B).
Conclusion:
A novel head array coil by integrating 4-channel 2H loop coils and 4-channel 1H microstrip elements reported in this work provides excellent DMRSI and proton signal coverage in a head-size lightbulb-shaped phantom. Each of the 2H coil element shows similar and excellent performance regarding Q factor, nominal 90o voltages and noise level; and is well decoupled to each other. This multinuclear array coil will be highly valuable for imaging human brain glucose metabolism at 7T under normal and diseased state.Acknowledgements
This work was supported in part by NIH grants of R01 CA240953, U01 EB026978, R01 MH111413, P41 EB027061.References
1. Lu, M., et al., Quantitative assessment of brain glucose metabolic rates using in vivo deuterium magnetic resonance spectroscopy. J Cereb Blood Flow Metab, 2017. 37(11): p. 3518-3530.
2. De Feyter, H.M., et al., Deuterium metabolic imaging (DMI) for MRI-based 3D mapping of metabolism in vivo. Sci Adv, 2018. 4(8): p. eaat7314.
3. Li, Y, et al., Machine Learning-Enabled High-Resolution Dynamic Deuterium MR Spectroscopic Imaging. IEEE Trans Med Imaging doi:10.1109/TMI.2021.3101149 (2021).
4. Ruhm, L., et al., Deuterium metabolic imaging in the human brain at 9.4 Tesla with high spatial and temporal resolution. Neuroimage, 2021. 244: p. 118639.
5. Avdievich, N.I., et al., Resonant inductive decoupling (RID) for transceiver arrays to compensate for both reactive and resistive components of the mutual impedance. NMR Biomed, 2013. 26(11): p. 1547-54.
6. Zhang, X., et al., Microstrip RF surface coil design for extremely high-field MRI and spectroscopy. Magn Reson Med, 2001. 46(3): p. 443-50.
Figures