4488
Intravoxel Architecture Atlases (IAA) Across the Human Lifespan
Ye Wu1, Sahar Ahmad1, and Pew-Thian Yap1
1Department of Radiology and Biomedical Research Imaging Center (BRIC), University of North Carolina at Chapel Hill, Chapel Hill, NC, United States
1Department of Radiology and Biomedical Research Imaging Center (BRIC), University of North Carolina at Chapel Hill, Chapel Hill, NC, United States
Synopsis
Human brain atlases integrate multifaceted features of the brain in common coordinate spaces, allowing systematic investigation of brain development and maturation. Here, we introduce a set of intravoxel architecture atlases (IAA) covering changes of tissue microstructure from birth to 100 years of age.
Introduction
The human brain is arguably the most complex organ in the body, yet its macroscopic layout is nearly complete at the time of term birth1. Postnatally, the brain develops rapidly from infancy through adolescence, stabilizes during young and middle adulthood, and degenerates in late adulthood. The precise charting of human brain development and aging is now possible due to data collected via the Lifespan Human Connectome Project (HCP), which includes (i) Baby Connectome Project (BCP2, 0-5 years); (ii) HCP Development (HCP-D3, 6-21 years); (iii) HCP Young Adult (HCP-YA4, 22-37 years); and (iv) HCP Aging (HCP-A5, 36-100 years). Leveraging these unique datasets, Ahmad et al.6 constructed a set of anatomical atlases covering the entire human lifespan from birth to 100 years of age. Although these atlases characterize the structural growth of the brain, lifespan atlases characterizing developmental trajectories of tissue microstructure remain elusive.Here, we introduce a set of intravoxel architecture atlases (IAA) that cover birth to 100 years of age, characterizing (i) isotropic restricted diffusion; (ii) free water; (iii) anisotropic restricted diffusion; and (iv) fiber orientation distribution functions to facilitate quantification of microstructural changes across the human lifespan. To our knowledge, this is to date the first work on constructing lifespan intravoxel architecture atlases, providing a complete picture of microstructural development in 100 years of postnatal life.
Materials and Methods
We pooled diffusion MRI (dMRI) data from BCP, HCP-D, HCP-YA, and HCP-A. All diffusion-weighted images were corrected for eddy-current and susceptibility distortions. We quantified intravoxel architecture via super-resolution asymmetry spectrum imaging (SR-ASI)7–9. SR-ASI fits a mixture of asymmetric fiber orientation distribution functions (FODFs) to the diffusion signal and estimates the tissue microstructure configuration that best explains the signal. Scalar indices characterizing tissue microstructure were computed via the fitted SR-ASI model8. SR-ASI allows a wide variety of features, such as the intracellular volume fraction, extracellular volume fraction, free-water volume fraction, and anisotropy index, to be computed for comprehensive microstructural analysis. In addition, SR-ASI characterizes fine-scale to coarse-scale diffusion spectrum and is hence well suited for capturing dynamic spatiotemporal microstructural changes in the human brain.In this work, we focus on constructing the atlases with four components: (a) Isotropic restricted diffusion10–12 - Water movement restricted equally in any direction; (b) Free water13 - Extracellular non-flowing water; (c) Anisotropic restricted diffusion10–12 - Restriction of water molecules with directional preference; and (d) Fiber orientation distribution functions (FODFs)7–9 - Local axonal orientations that can be utilized for diffusion tractography to estimate white matter pathways.
We constructed the atlases at 14 time points. The components of each subject are warped to the atlas space via a transform that is estimated with both rigid and non-linear registration between the tissue map of the subject and the age-specific atlas. Following the atlas construction method6, the warped components of each subject within a given time interval are combined via weighted averaging to obtain an intravoxel architecture atlas. FODF reorientation was performed with apodized point spread functions14 before averaging to create the FODF atlas.
To quantify microstructure changes over the lifespan, tissue anisotropy15 was computed as the square root of the power of the l=2 band of the FODF. The mean tissue anisotropy for each age group was also calculated.
Results
Our dataset consisted of 425 scans. Figure 1 shows the distribution of the subjects used in the construction of the age-specific atlases. Figure 2 shows the age-specific volumetric atlases for isotropic restricted diffusion, free water, anisotropic restricted diffusion, and fiber orientation distribution functions. Figure 3 indicates an increasing then decreasing trend for the WM volume. However, a heterogeneous development trend for the tissue anisotropy was found beyond 3 years, suggesting a different developmental pattern between brain volume and tissue anisotropy. The brainstem acts as a relay center connecting the cerebrum and cerebellum to the spinal cord, and is typically characterized by complex fiber configurations. As an example, Figure 4 shows local development within the brainstem, indicating clear changes of the medial longitudinal fasciculus from birth to 100 years of age. This is in line with the observation that the tissue microstructural properties of the brainstem evolve across the lifespan16.Conclusion
We presented a set of lifespan human brain atlases that capture microstructural changes of the human brain, providing consistent reference templates.Acknowledgements
This work was supported in part by the United States National Institutes of Health (NIH) through grants MH125479, EB008374, and EB006733. Data were provided by the Human Connectome Project, WU-Minn Consortium (Principal Investigators: David Van Essen and Kamil Ugurbil; 1U54MH091657) funded by the 16 NIH Institutes and Centers that support the NIH Blueprint for Neuroscience Research; and by the McDonnell Center for Systems Neuroscience at Washington University.References
1. Dobbing J, Sands J. Quantitative growth and development of human brain. Arch Dis Child. 1973;48:757–767.2. Howell BR, Styner MA, Gao W, et al. The UNC/UMN Baby Connectome Project (BCP): An overview of the study design and protocol development. Neuroimage. 2019;185:891–905.
3. Somerville LH, Bookheimer SY, Buckner RL, et al. The Lifespan Human Connectome Project in Development: A large-scale study of brain connectivity development in 5–21 year olds. Neuroimage. 2018;183:456–468.
4. Van Essen DC, Smith SM, Barch DM, Behrens TEJ, Yacoub E, Ugurbil K. The WU-Minn Human Connectome Project: An overview. NeuroImage 2013. p. 62–79.
5. Bookheimer SY, Salat DH, Terpstra M, et al. The Lifespan Human Connectome Project in Aging: An overview. Neuroimage. 2019;185:335–348.
6. Ahmad S, Wu Y, Wu Z, et al. Human Brain Surface-Volume Atlases of Newborns to Centenarians. The 27th Annual Meeting and Educational Courses of OHBM 2021. 2021.
7. Wu Y, Hong Y, Ahmad S, et al. Globally Optimized Super-Resolution of Diffusion MRI Data via Fiber Continuity. Medical Image Computing and Computer Assisted Intervention – MICCAI 2020. Springer, Cham; 2020. p. 260–269.
8. Wu Y, Lin W, Shen D, Yap P-T, Consortium UBCP, Others. Asymmetry Spectrum Imaging for Baby Diffusion Tractography. International Conference on Information Processing in Medical Imaging. Springer, Cham; 2019. p. 319–331.
9. Wu Y, Hong Y, Feng Y, Shen D, Yap P-T. Mitigating Gyral Bias in Cortical Tractography via Asymmetric Fiber Orientation Distributions. Med Image Anal. Elsevier; 2020;59:101543.
10. Mukherjee P, Chung SW, Berman JI, Hess CP, Henry RG. Diffusion Tensor MR Imaging and Fiber Tractography: Technical Considerations [online]. American Journal of Neuroradiology 2008. p. 843–852.
11. White NS, Leergaard TB, D’arceuil H, Bjaalie JG, Dale AM. Probing tissue microstructure with restriction spectrum imaging: Histological and theoretical validation. Hum Brain Mapp. Wiley Online Library; 2013;34:327–346.
12. Huynh KM, Xu T, Wu Y, et al. Probing Tissue Microarchitecture of the Baby Brain via Spherical Mean Spectrum Imaging. IEEE Trans Med Imaging. 2020;39:3607–3618.
13. Pasternak O, Sochen N, Gur Y, Intrator N, Assaf Y. Free water elimination and mapping from diffusion MRI. Magn Reson Med. 2009;62:717–730.
14. Raffelt D, Tournier J-D, Crozier S, Connelly A, Salvado O. Reorientation of fiber orientation distributions using apodized point spread functions. Magn Reson Med. 2012;67:844–855.
15. Wilson S, Pietsch M, Cordero-Grande L, et al. Development of human white matter pathways in utero over the second and third trimester. Proc Natl Acad Sci U S A [online serial]. National Academy of Sciences; 2021;118.
16. Bouhrara M, Cortina LE, Khattar N, et al. Maturation and degeneration of the human brainstem across the adult lifespan. Aging . 2021;13:14862–14891.
Figures
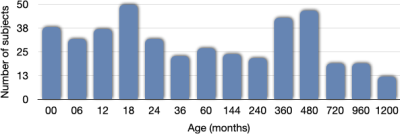
Fig. 1. The number of subjects used for constructing the atlas for each age group.
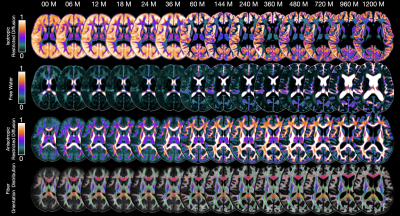
Fig. 2. Intravoxel architecture atlases from birth to 100 years of age.
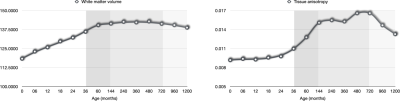
Fig. 3. White matter volume and FODF-based tissue anisotropy.
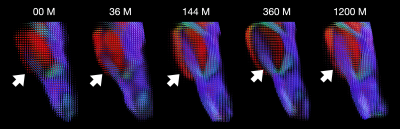
Fig. 4. Fiber orientation distribution functions within the brainstem at five time points.
DOI: https://doi.org/10.58530/2022/4488