4486
Adaptive-CS-Net to Accelerate 3D T1-weighted Imaging: Brain Volume Measures for clinical use
Sandeep Ganji1,2, Brian Johnson3,4, John Penatzer3, and Johannes M. Peeters5
1MR R&D, Philips Healthcare, Rochestr, MN, United States, 2Mayo Clinic, Rochester, MN, United States, 3Philips Healthcare, Gainesville, FL, United States, 4University of Texas Southwestern Medical Center, Dallas, TX, United States, 5Philips Healthcare, Eindhoven, Netherlands
1MR R&D, Philips Healthcare, Rochestr, MN, United States, 2Mayo Clinic, Rochester, MN, United States, 3Philips Healthcare, Gainesville, FL, United States, 4University of Texas Southwestern Medical Center, Dallas, TX, United States, 5Philips Healthcare, Eindhoven, Netherlands
Synopsis
Despite long scan times, 3D T1-weighted (T1w) MRI is routinely used for MRI studies to provide high resolution structural and volumetric information of brain. Volumetric analysis can serve as a biomarker and aid in clinical diagnosis of certain diseases such as Alzheimer’s, mild cognitive impairment, and atrophy, however, the standardized 3D T1-weighted scans suffer from long acquisition times well over 5 minutes. We compared the results of volumetric brain analysis for 3D T1w images acquired over a range of compressed SENSE acceleration factors with and without Adaptive-CS-Net reconstruction against a standard clinical 3D T1w MRI protocol.
Introduction
Despite long scan times, 3D T1-weighted (T1w) MRI is routinely used for MRI studies to provide high resolution structural and volumetric information of brain. Volumetric analysis can serve as a biomarker and aid in clinical diagnosis of certain diseases such as Alzheimer’s, mild cognitive impairment, and atrophy, however, the standardized 3D T1-weighted scans suffer from long acquisition times well over 5 minutes. Recent advances in image acceleration and deep learning-based reconstruction (1 - 5) provide promise for drastically decreasing acquisition times while maintaining image quality for accurate diagnosis. Prior studies have already shown the utility of compressed SENSE and deep-learning based acceleration (1, 5 - 6). Here we compare the results of volumetric brain analysis for 3D T1w images acquired over a range of compressed SENSE acceleration factors with and without Adaptive-CS-Net reconstruction (6) against a standard clinical 3D T1w MRI protocol.Methods
Five healthy male subjects (38±6 years) were scanned at 3T (Philips Elition X, Philips Healthcare, Best, Netherlands) using a 32-channel head coil. Standard clinical protocol 3D T1w images were acquired using a FOV=256 x 256 x 192 mm3 (160 slices at acquisition resolution of 1.2 x 1.2 x 1.2 mm3) with a SENSE acceleration factor of 1.8 (along the RL direction). Using the same resolution, additional 3D T1w images were acquired with increasing compressed SENSE acceleration factors of 1.8 up to 14.4. More details about the MRI acquisition protocol can be found in Figure 1. Scans using compressed SENSE were reconstructed using vendor provided compressed reconstruction on the scanner and with Adaptive-CS-Net artificial intelligence framework (6). Volumetric segmentation was performed using FastSurfer (7) and FreeSurfer (8) software tools, which are documented and freely available for download online (https://github.com/Deep-MI/FastSurfer and http://surfer.nmr.mgh.harvard.edu/, respectively).Results
All acquired images and accelerations were able to be successfully post-processed using the FastSurfer and FreeSurfer software tools and volumetric data were generated. Figure 2 shows a sample of the image quality generated as a function of increasing compressed SENSE acceleration factors, including the clinical standard image. Visual inspection images showed higher noise as the compressed SENSE acceleration increased, however with Adaptive-CS-Net reconstruction some of the noise was reduced. Contrasts for signal to noise and artifacts show adequate image quality up to an acceleration factor of 14.4 compressed SENSE acceleration factor. Figure 3 shows the volumetric data from few selected brain regions, in a Bland-Altman plot, using standard clinical protocol 3D T1w with SENSE acceleration factor of 1.8 as reference. Overall, the volumetric variation with increasing compressed SENSE acceleration factors was within 5% the standard clinical protocol 3D T1w. Moreover, the consistency for large regions volumes, such as cerebral-white matter and hippocampus was much narrower compared to small regions, as expected. The heatmap of the averaged data from 5 subjects shown in Figure 4, demonstrates the variation with respect to standard clinical protocol 3D T1w with SENSE. The deviation from the standard clinical protocol 3D T1w values increases only at compressed SENSE acceleration factor of 14.4.Discussion
Here we applied compressed SENSE with factors up to 14.4 acquire 3D T1w images to evaluate the effect on volumetric analysis. Applying an compressed SENSE acceleration factor of 7.2 produced a 1 minute and 12 seconds scan time with no evidence of significant differences in volumetric results. Pushing to a compressed SENSE acceleration to 14.4 yielded generated image contrasts with a higher noise profile but still clinically diagnostic for a scan time of only 37 seconds. Even at compressed SENSE acceleration factor of 14.4, the volumetric results were under 5% different when compared to the volumetric results standard clinical protocol 3D T1w images. Images acquired with compressed SENSE acceleration factors with and without Adaptive-CS-Net reconstruction showed slight variation, which may need to be further investigated in a larger study.Conclusion
Compressed SENSE based acceleration can be used to achieve clinically viable scan times while maintaining image quality and providing the volumetric information within 5% of the values of the standard clinical protocol 3D T1w. Drastically lowering the acquisition time to scans below 2-minutes could lead to more widespread clinical adoption of incorporating volumetric based measurements.Acknowledgements
No acknowledgement found.References
- Lustig M, Donoho D, Pauly JM. Sparse MRI: The application of compressed sensing for rapid MR imaging. Magn Reson Med. 2007 Dec;58(6):1182-95. doi: 10.1002/mrm.21391. PMID: 17969013.
- Wang S, Su Z, Ying L, Peng X, Zhu S, Liang F, et al., “Accelerating Magnetic Resonance Imaging via Deep Learning,” IEEE Conference on International Symposium on Biomedical Imaging (ISBI), pp. 514–517, 2016.
- Liang D, Cheng J, Ke Z, Ying L. Deep Magnetic Resonance Image Reconstruction: Inverse Problems Meet Neural Networks. IEEE Signal Process Mag. 2020;37(1):141-151. doi:10.1109/MSP.2019.2950557
- T. M. Quan, T. Nguyen-Duc and W.-K. Jeong, "Compressed sensing MRI reconstruction using a generative adversarial network with a cyclic loss", IEEE Trans. Med. Imag., vol. 37, no. 6, pp. 1488-1497, Jun. 2018.
- M. Mardani, E. Gong, J. Y. Cheng, S. S. Vasanawala, G. Zaharchuk, L. Xing, et al., "Deep generative adversarial neural networks for compressive sensing MRI", IEEE Trans. Med. Imag., vol. 38, no. 1, pp. 167-179, Jan. 2019.
- N. Pezzotti et al., "An Adaptive Intelligence Algorithm for Undersampled Knee MRI Reconstruction," in IEEE Access, vol. 8, pp. 204825-204838, 2020, doi: 10.1109/ACCESS.2020.3034287.
- Henschel L, Conjeti S, Estrada S, Diers K, Fischl B, Reuter M, FastSurfer - A fast and accurate deep learning based neuroimaging pipeline, NeuroImage 219 (2020), 117012. https://doi.org/10.1016/j.neuroimage.2020.117012
- Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. 1999 Feb;9(2):179-94. doi: 10.1006/nimg.1998.0395. PMID: 9931268.
Figures
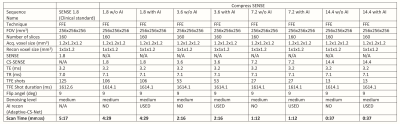
MRI protocol imaging parameters
Sample of the 3D T1-weighted (T1w) images
generated from Standard clinical protocol 3D T1w, multiple compressed SENSE
(CS) acceleration factors with standard and Adaptive-CS-Net
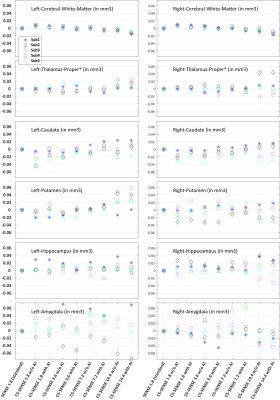
Bland–Altman plot of the volumetric data using 3D T1w images acquired with SENSE acceleration factor of 1.8 as reference.
Heatmap plot of the 84 brain regions volumetric data averaged across 5 subjects (42 from each of the hemisphere).
DOI: https://doi.org/10.58530/2022/4486