4482
MR imaging of increased fibrotic tissue and hyaluronan deposition in the advanced stages of pancreatic ductal adenocarcinoma1Radiology, University of Washington, Seattle, WA, United States, 2Applied Physics Laboratory, University of Washington, Seattle, WA, United States, 3Gastroenterology, University of Washington, Seattle, WA, United States, 4Arts and Sciences, University of Washington, Seattle, WA, United States, 5Urology, University of Washington, Seattle, WA, United States
Synopsis
Pancreatic cancer is expected to become the second leading cause of the cancer-related deaths in the USA by 2030. A genetically engineered mouse model (KPC) offers an alternative to transplantation models for preclinical therapeutic evaluation as it expresses mutations similar to human pancreatic cells. MRI can be used to monitor the tumor microenvironment in a variety of tumors. The goal of this study was to monitor fibrotic tissue and hyaluronan deposition in the KPC mouse model during the late stages of tumor development.
Purpose
Recently, a genetically engineered mouse model (KPC) has been developed that offers an alternative to transplantation models for preclinical therapeutic evaluation as it expresses mutations similar to human pancreatic cells [1] and develops pancreatic tumors whose pathophysiology and molecular features resemble those of human pancreatic ductal adenocarcinoma (PDAC) [2]. Multi-parametric magnetic resonance imaging (mp-MRI) has yielded good results in pancreatic cancer detection, location in monitoring therapeutic effects [3, 4]. Previously, we have demonstrated changes in the tumor microenvironment (TME) in tumors less than 250 mm3. The purpose of this study was to monitor the accumulation of fibrotic tissue and hyaluronan using MRI in the late stages of the tumor.Methods
KPC mice (n = 24) were enrolled in the study when they had a tumor mass > 250 mm3, confirmed by ultrasound imaging and MR imaging. All mice were euthanized at the terminal time point and the tumor and associated pancreatic tissue was excised and prepared for histological evaluation. Anatomical images: The multi-slice MRI protocol, covering the whole tumor, started with fat-suppressed T1 weighted coronal images (repetition time (TR) = 2000 ms, echo time (TE) = 5.49 ms, field of view (FOV) = 30 × 30 mm, matrix size = 128 × 256; for anatomical reference. Subsequently, 20 axial images were acquired to calculate tumor volume. T1 weighted images were acquired using following parameters: TE = 9.66 ms, TR = 5500, 3000, 1500, 1000, 385.8 ms, NA = 1, spatial resolution of 0.117 x 0.234 mm/pixel. The quantitative T2 maps were generated using a multi-slice multi echo sequence, with fat signal suppressed, utilizing following parameters: TR = 4000 ms; TE = 12 echoes equally spaced from 6.28 ms to 75.4 ms; NA = 1. Diffusion weighted images were acquired with 8 different b values (0, 30, 60, 100, 150, 200, 300, 500) s/mm2. Low b values (i.e., <100 s/mm2 were used to generate perfusion fraction maps, and higher b values (i.e., >100 s/mm2 were used to generate tissue diffusivity maps. Magnetization transfer ratio (MTR %) images were acquired using a gradient echo sequence (TR/TE = 625/2 ms, flip angle =30o) with an off-resonance frequency of 7000 Hz and a saturation pulse block pulse shape, 50 ms width, and 10 µT amplitude. A series of 10 images were acquired with FOV = 30 x 30 mm, matrix size = 256 x 256 yielding spatial resolution of 0.117 x 0.117 mm/pixel. On a single 1 mm slice, delineating tumor, amide proton transfer (APT) imaging was performed. Chemical exchange saturation transfer (CEST) imaging data were acquired using RARE sequence (continuous-wave block pulse, B1 = 0.5 μT, duration = 2 s), which was applied at 25 frequency offsets from − 360 Hz to 360 Hz with an interval of 0.5 ppm to estimate a center frequency shift (water saturation shift referencing (WASSR) approach). Other imaging parameters were: TR/TE = 2200/7 ms, FOV = 30 × 30 mm, matrix size = 128 × 128, flip angle = 180o, and number of excitations = 1. For saturation a single slice was acquired with 6 frequency offsets at ±3.0, ±3.5, ±4.0 ppm, with an off-resonance RF pulse applied for 3 s at a power level of 2 μT. Other parameters were: TR/TE = 5000/7 ms, matrix = 128 × 128 (reconstructed phase encoding steps = 128; acquisition phase encoding steps = 96), FOV = 30 × 30 mm, rare factor = 8. Finally, a control image with the saturation offset at 300 ppm was also acquired. All data were analyzed using Image J ((http://imagej.nih.gov/ij/), and Horos software (v 3.3.6, https://horosproject.org/). Histological image analysis: All histological image analysis was done using a custom script written for MATLAB (2020b, The Mathworks, Natick, MA).Results
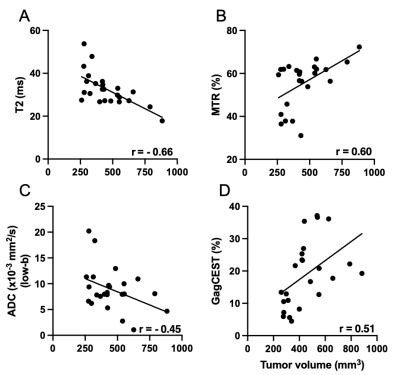
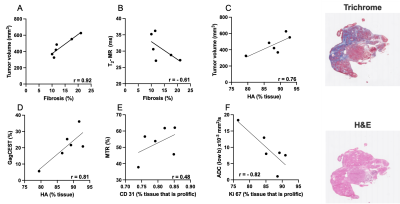
We demonstrate here that there was a significant correlation between tumor volume and T2 (r = -0.66), MTR (r = 0.60), apparent diffusion coefficient (ADC)_low b (r = 0.62), and Gag-CEST (r = 0.51) (Figure 1). A subset of mice was randomly selected for histological and IHC analysis. There was a positive correlation between tumor volume and fibrotic tissue accumulation (r = 0.92), and hyaluronan (or hyaluronic acid or HA) (r = 0.76); Gag-CEST and HA (r = 0.81), and MTR and CD31 cells (r = 0.48) and a negative correlation between ADC_low b (perfusion) and Ki67 (r = 0.82) (Figure 2).Conclusions
In this study we have demonstrated decrease in T2 and increase in MTR (%) in the late stages of tumor development suggesting accumulation of fibrotic tissue. Tumor perfusion decreased and hyaluronan deposition increased simultaneously with tumor progression.Acknowledgements
No acknowledgement found.References
1. Hingorani, S. R., Wang, L., Multani, A. S., Combs, C., Deramaudt, T. B., Hruban, R. H., Rustgi, A. K., Chang, S., Tuveson, D. A. Trp53R172H and KrasG12D cooperate to promote chromosomal instability and widely metastatic pancreatic ductal adenocarcinoma in mice. Cancer Cell. 2005;7(5):469-483.
2. Hruban, R. H., Adsay, N. V., Albores-Saavedra, J., Anver, M. R., Biankin, A. V., Boivin, G. P., Furth, E. E., Furukawa, T., Klein, A., Klimstra, D. S., Kloppel, G., Lauwers, G. Y., Longnecker, D. S., Luttges, J., Maitra, A., Offerhaus, G. J., Perez-Gallego, L., Redston, M., Tuveson, D. A. Pathology of genetically engineered mouse models of pancreatic exocrine cancer: consensus report and recommendations. Cancer Res. 2006;66(1):95-106.
3. Maloney, E., DuFort, C. C., Provenzano, P. P., Farr, N., Carlson, M. A., Vohra, R., Park, J., Hingorani, S. R., Lee, D. Non-Invasive Monitoring of Stromal Biophysics with Targeted Depletion of Hyaluronan in Pancreatic Ductal Adenocarcinoma. Cancers (Basel). 2019;11(6).
4. Vohra, R., Park, J., Wang, Y. N., Gravelle, K., Whang, S., Hwang, J. H., Lee, D. Evaluation of pancreatic tumor development in KPC mice using multi-parametric MRI. Cancer imaging: the official publication of the International Cancer Imaging Society. 2018;18(1):41.
Figures