4477
Pulmonary Vasculature and T2* mapping of Lung in post-COVID subjects using Free-Breathing 3D UTE
Vadim Malis1, Won Bae1, Jirach Kungsamutr2, Xiaowei Zhang1, Yoshimori Kassai3, Andrew Yen1, Susan Hopkins1, Yoshiharu Ohno4, and Mitsue Miyazaki1
1Radiology, UC San Diego, San Diego, CA, United States, 2Bioengineering, UC San Diego, San Diego, CA, United States, 3Canon Medical Systems Corp., Tochigi, Japan, 4Radiology, Fujita Health University, Toyoake, Japan
1Radiology, UC San Diego, San Diego, CA, United States, 2Bioengineering, UC San Diego, San Diego, CA, United States, 3Canon Medical Systems Corp., Tochigi, Japan, 4Radiology, Fujita Health University, Toyoake, Japan
Synopsis
Free-breathing 3D MRI techniques are applied to explore the impact of COVID-19 in adults. Single echo non-fat suppressed Ultra-short TE (UTE) was utilized for automatic segmentation of lung, Multi-echo UTE with fat suppression was used to obtain T2* maps, and UTE with Time-Spatial Labeling Inversion Pulse (Time-SLIP) allowed to visualize pulmonary vasculature.
Introduction
As of November 2021, over 240M people have suffered severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) disease, resulting in over 5M deaths. Most healthy people recover within a few weeks, but as many as 30% continue to experience post-COVID-19 symptoms that linger over time including fatigue, cough, shortness of breath, headache, and joint pain, termed long COVID (1). MRI can make a valuable contribution in studying the impact of COVID-19 on lungs. Use of Ultra-short TE (UTE) imaging with TE of less than 0.1 ms allows visualization of lung parenchyma despite low proton density and short T2*(2). In this study, we examined the T2* maps and pulmonary vasculature of healthy and post-COVID subjects.Methods
Three healthy (38 ± 8 years) and two post-COVID (50 ± 8 years) subjects confirmed with COVID-19 PCR test were scanned on a clinical 3T imager (Vantage Galan 3T, Canon Medical Systems, Japan) after obtaining IRB-approved written informed consent. Images were acquired using body SPEEDER and spine SPEEDER coils. The scanning protocol included the following series: (a) 3D UTE without fat suppression (TE/TR = 96 μs/3.7 ms, NEX = 1, FA = 5°); (b) multi-echo UTE (six TEs = 96 μs / 2.3/4.5/6.7/8.9/11.1 ms, with fat suppression: five SPectral Adiabatic Inversion Recovery (SPAIR) pulses per 64 segments were applied, resulting in approximately one SPAIR per 36 UTE lines, TR = 16.9ms, NEX = 1, FA = 4°); (c) Time-Spatial Labeling Inversion Pulse (Time-SLIP) tagged 3D UTE (TI = 500, 1000, and 1500 ms, TR = 3.7 ms, TE = 96 μs, NEX = 1, FA = 5°. All study series were acquired with the respiratory bellows during the expiratory phase of the cycle and shared the same geometric parameters: coronal orientation, FOV = 40×40 cm, matrix size 256×256. Image series (a) was utilized for the automated lungs segmentation using a set of morphological operations: a.1) finding two largest areas with a low signal at each slice, a.2) dropping voxels with less than 13 nearest neighbor connections, a.3) the resultant lung surface was smoothed with a 3Dal gaussian kernel. T2* maps were estimated for the segmented region from multi-echo UTE data (b) using in-house built software with multithreading support (3). The lung region from Time-SLIP 3D UTE images (c) was visualized by plotting Maximum Intensity Projections (MIP) for each of the TI.Results
Images of all six echoes of 3D UTEs for a selected coronal slice in one of the healthy subjects are shown in Figure 1. Instead of collecting 6 different echoes with separate image series, a single acquisition of about 8 minutes was performed, the actual scan time depends on the respiratory rate. The first TE is a minimum TE (96 μs), and the consecutive echoes are selected to be in-phase (~2.2 ms at 3 T) cycles to avoid interference of fat signals. Figure 2 shows the voxel-wise T2* colormaps of lungs superimposed over the first echo of 3D UTE image for two healthy (left panel) and two post-COVID-19 (right panel) subjects. The average T2* of the entire lungs of the healthy subjects was 1.09 ± 0.21 ms which is similar to the previous reports(4, 5). In post-COVID-19 subjects, multiple regions with abnormally long T2* were observed. Figure 3 animates changes in MIP images with changes in TI. Less perfusion at the superior lobe of right lung in the post-COVID-19 subject was observed. A 10-cm wide Time-SLIP tag pulse (represented with the green rectangles) was applied on the heart. Simplistic pulse sequence diagram is shown at the bottom. Note that TI period is measured from the middle of the tag pulse to the start of read-out time. UTE acquisition window is about 950 ms and considering the contrast TIc = TI + 475 ms. Resultant TI of 500 ms before the 3D UTE readout acquisition allows tagged blood to travel from the heart and reach pulmonary arteries and branches. At TI of 1500 ms, periphery regions of lung vessels are depicted. The TI periods of 500, 1000, 1500 ms are TIc of 975, 1475, and 1975 ms, respectively.Discussion
In this study, both post-COVID-19 subjects had frequent cough and shortness of breath, who had long T2* signals of about 30-40 ms in their lung parenchyma. Our hypothesis is that the short T2* signal from lung parenchyma might be contaminated with long T2* component of mucus in the bronchioles and alveoli for the post-COVID-19 subjects. The perfusion images of healthy subjects have shown more detailed homogenous vasculature compared to the post-COVID-19 subjects.Conclusions
Free-breathing 3D UTE MR imaging techniques successfully provide visualization of pulmonary vasculature and T2* mapping of lungs in post-COVID-19 adults. Future studies may incorporate scans at inspiration to measure lungs volume change and specific ventilation with the ultimate goal to establish a comprehensive “one-stop-shopping” MR methodology to monitor lung functions during the post-COVID-19 recovery.Acknowledgements
Authors acknowledge the grant support from Canon Medical Systems Corp., Japan.References
[1] L. Huang et al., Lancet. 398, 747–758 (2021).
[2] C. J. Bergin et al., Radiology. 179, 777–781 (1991).
[3] V. Malis et al., in ISMRM (2021).
[4] Y. Ohno et al., Am J Roentgenol. 197, W279–W285 (2011).
[5] N. D. Gai et al., J Magn Reson Imaging. 45, 1097–1104 (2017).
Figures
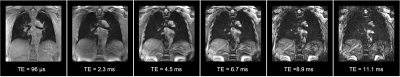
Figure 1. Coronal slices of all six echoes acquired in 3D multi-echo UTE from one of the healthy subjects.
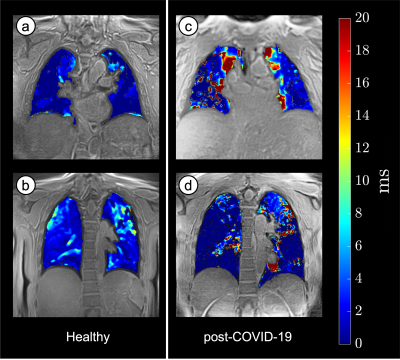
Figure 2. T2* colormaps of the healthy (A, B) and post-COVID-19 (C, D) lungs overlayed on the UTEimage. Note that healthy lungs show T2* of 1-2 ms, while the post-COVID-19 have regions with T2* of 30-50 ms.
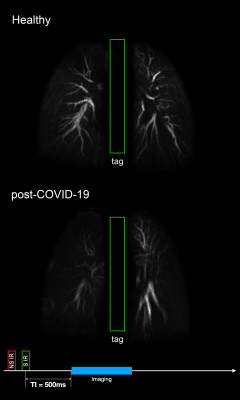
Figure 3. Non-contrast MR perfusion maximum intensity projection (MIP) of a healthy non-COVID-19 subject and post-COVID-19 subject using Time-SLIP 3D UTE with TI of 500, 1000, and 1500 ms. Note that less perfusion in the post-COVID-19 subject, especially at the right upper lung. The 10-cm wide Time-SLIP tag pulse was applied on the heart, represented with the green rectangles. Simplistic pulse sequence diagram is shown at the bottom.
DOI: https://doi.org/10.58530/2022/4477