4474
Compressed sensing improved the assessment of the corticospinal pathways in stroke brains scanned with traditional DTI1Yerkes Imaging Center, Yerkes National Primate Research Center, Emory University, Atlanta, GA, United States, 2Division of Neuropharmacology and Neurologic Diseases, Yerkes National Primate Research Center, Emory Univeristy, Atlanta, GA, United States
Synopsis
Conventional diffusion tensor imaging (DTI) has been widely used to examine white matter connectivity with neurological diseases but is limited in detecting pathways with crossing fibers. As the crossing fibers are abundant in large and complicated brains like human and non-human primate brains, the DTI tractography results may be biased substantially. In stroke research, the integrity of corticospinal tract (CST) plays a critical role in assessing the motor function. In this study, a compressed sensing technique was explored to improve the CST delineation with conventional DTI, and the feasibility was evaluated by using a monkey model of stroke.
Introduction
DTI is a powerful tool to examine the white matter integrity and brain connectivity but hindered by limited angular resolution to delineate the pathways with crossing fibers. Although Q-ball imaging technique can resolve this issue by using a large number of diffusion-encoding directions 1, its application could be limited in preclinical and clinical studies due to increasing scanning time and demand of high b values. The compressed sensing (CS) technique has demonstrated its potential to improve the tractography of heathy human brains scanned with traditional DTI and high angular resolution diffusion imaging (HARDI) 2-3. As the corticospinal tract (CST) plays a critical role in motor function and can be impaired by stroke, the stroke patients and animal models are still widely scanned with traditional DTI 4. In the present study, the compressed sensing technique was explored to assess the detection ability of progressive alterations of the CST in the stroke monkey brains scanned with conventional DTI.Methods
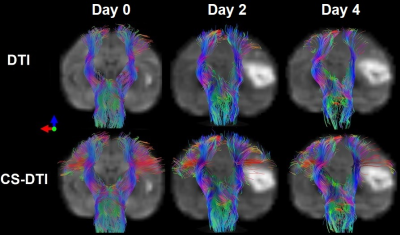
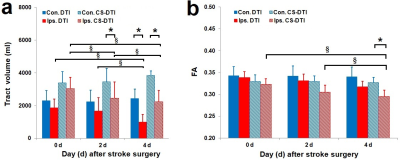
Healthy adult rhesus monkeys (n=4, 10-21 years old) was induced with permanent middle cerebral artery (MCA) occlusion. DTI was conducted on Day 0 (within 6 hours), Day 2 and Day 4 post occlusion on a 3T scanner. Animals were sacrificed immediately after their last MRI scans for necropsy. The DTI data were acquired with a single-shot EPI sequence with TE/TR = 80 ms/5 s, data matrix = 64 × 64, isotropic resolution = 1.5 mm, slice thickness = 1.5 mm, 30 gradient directions with b-value 1000 s/mm2. Structural T2-weighted images were collected to identify the brain structure and infarction evolution. DTI data were analyzed with conventional DTI tractography 5, and further processed with the CS-based DTI approach (CS-DTI) by using the crossing fiber angular resolution of intra-voxel structure (CFARI) algorism 3. A region-of-interest (ROI) was separately placed in the left or right medulla manually drawn on the b0 diffusion-weighted images, and the corticospinal fibers passing through each ROI were selected. The tract volume of the CST was extracted for each hemisphere and examined seperately. A two-way multivariate analysis of variance was performed for the tract volume and the calculated fractional anisotropy (FA) in the CST delineated with traditional DTI and CS-DTI tractography, followed by post hoc comparisons between the two hemispheres (contralateral vs. ipsilateral) or across all the time points (0, 2 and 4 days) post stroke with Bonferroni correction.Results
The tractography and statistical results were illustrated in Figure 1 and Figure 2 respectively. By using conventional DTI tractography, reduction of the tract volumes in the CST were seen in the ipsilateral hemisphere compared to those in the contralateral hemisphere 4 days post occlusion (p < 0.05). Also, no significant difference was observed in FA of the CST between the two hemispheres at any time point post occlusion (p > 0.1). In contrast, by using CS-DTI tractography, the tract volume reduction in the CST was observed 2 and 4 days post occlusion (p < 0.05), and FA reduction in the CST was seen on 4 days post-occlusion (p < 0.05). White matter lesion within or adjacent to the infarct areas on Day 2 and Day 4 was verified with Luxol fast blue stain 6.With conventional DTI, the tract volume of the CST in the ipsilateral hemisphere decreased significantly from Day 0 to Day 4 post occlusion (p < 0.01), and no significant FA change in the CST was seen between the time points post occlusion (p > 0.05). In contrast, by using CS-DTI tractography, decrease of the tract volume in the CST was observed from Day 0 to Day 2 post occlusion (p < 0.05), and FA reduction was seen from Day 0 to Day 4 post occlusion as well (p < 0.01).
Discussion and Conclusion
The preliminary results demonstrated that the corticospinal fibers missed by traditional DTI tractography could be substantially recovered by the compressed sensing enhanced tractography approach. The tract volume and FA in the CST derived from CS-DTI showed better sensitivity in detection of early corticospinal fiber degeneration following stroke insult in the brain compared to those from traditional DTI tractography. In particular, our results showed the CST lesion could be observed on Day 2 by examining the tract volume with CS-DTI while it was seen on Day 4 with traditional DTI tractography, suggesting the compressed sensing based fiber tracking approach may be useful to enhance the assessment of the early damage or remodeling in the CST due to stroke insult.CST degeneration has been seen in stroke patients 7. Large animal models like non-human primates (NHPs) show greater translational potential in stroke research due to their similarity in brain anatomy and physiology to human compared to rodent models, and DTI is still widely used in studies of patients and animal models for its robustness and simplicity 8. Our preliminary results suggest the CS-DTI tractography algorithm could improve the sensitivity for detecting the CST injury in stroke brains and may be useful to exploit the regular DTI data for comprehensively understanding the CST integrity in stroke animals and patients and benefit the diagnosis of motor functions in the stroke research.
Acknowledgements
No acknowledgement found.References
1. Tuch DS. Q-ball imaging. Magn Reson Med. 2004;52(6):1358-1372.
2. Michailovich O, Rathi Y. Fast and accurate reconstruction of HARDI data using compressed sensing. Med Image Comput Comput Assist Interv. 2010;13(Pt 1):607-614.
3. Landman BA, Bogovic JA, Wan HL, et al. Resolution of crossing fibers with constrained compressed sensing using diffusion tensor MRI. Neuroimage. 2012;59(3):2175-2186.
4. Ma C, Liu A, Li Z, et al. Longitudinal study of diffusion tensor imaging properties of affected cortical spinal tracts in acute and chronic hemorrhagic stroke. J Clin Neurosci. 2014;21(8):1388-1392.
5. Mori S, van Zijl PC. Fiber tracking: principles and strategies - a technical review. NMR Biomed. 2002;15(7-8):468-480.
6. Zhang X, Yan Y, Tong F, et al. Progressive assessment of ischemic injury to white matter using diffusion tensor imaging: a preliminary study of a macaque model of stroke. Open Neuroimag J. 2018;12:30-41.
7. Domi, T, deVeber G, Shroff M, et al. Corticospinal tract pre-wallerian degeneration: a novel outcome predictor for pediatric stroke on acute MRI. Stroke. 2009;40(3):780-787.
8. Thiebaut de Schotten M, Dell'Acqua F, Valabregue R, et al. Monkey to human comparative anatomy of the frontal lobe association tracts. Cortex. 2012;48(1):82-96.
Figures