4471
Study on Plasma BDNF Concentration and Rs-fMRI Characteristics Related to Motor Function1Radiology, The First Affiliated Hospital of Guangxi Medical University, Nanning, China, 2Radiology, ,Sichuan Cancer Hospital and Institute, Chengdu, China, 3Siemens Healthcare, Beijing, China
Synopsis
Results of our study showed that the level of plasma brain derived neurotrophic factor (BDNF) was positively correlated with cognitive function, and negatively correlated the changes in local brain network related to the destruction of motor and non-motor cortical areas in the hemispheres using rs-fMRI in capsular stroke (CS) group. Follow-up results demonstrated the mechanism of local dynamic network remodeling and its correlation with plasma BDNF concentration and clinical outcomes. It provides temporal and spatial information for the dynamic separation of brain networks in different stage of CS, and reflects the potential biological mechanism of proportional recovery.
Introduction
As a common type of ischemic stroke (IS) involving the pyramidal tract, capsular stroke (CS) often involves motor dysfunction, and its pathogenesis and functional recovery frame have not yet been determined A number of studies have proved that 70% of dyskinesias have a recovery rate, and the proportional recovery may reflect the underlying biological mechanism of recovery1. Modern studies have proved the role of genetics in the risk and prognosis of stroke. Among them, brain derived neurotrophic factor (BDNF) plays a role in neuroplasticity, neuroprotection and neurogenesis, which may provide new insights for stroke2,3. As an emerging direction in the field of brain science, dynamic analysis method is a powerful supplement to static measurement based on rest state fMRI (rs-fMRI), which can improve our understanding of brain remodeling and proportional recovery after stroke. This research calculated three dynamic indicators (dALFF, dfALFF, dReHo) based on rs-fMRI to explore the mechanism of dyskinesias in CS and functional remodeling after 30 days and the relationship between the change of dynamic indicators and clinical behavior.Method
A total of 102 patients with subacute ischemic stroke, including 39 patients with CS, were enrolled consecutively from August 2019 to December 2020. 98 healthy controls were matched in this study. All patients underwent MRI examination on the MAGNETOM Prisma 3T scanner (Siemens Healthcare,Erlangen, Germany), 64-channel phased array head coil within 7 days (D7, baseline) and 30 days (D30) after infarct onset, meanwhile the control group performed just once. Rs-fMRI parameters were as follows: TR/TE 2000/35ms, FOV 240mm×240mm, voxel size 2.6mm×2.6mm×3mm, and 186 time points without interval scanning, acquisition time 6min20s. For each patient, National Institutes of Health Stroke Scale (NIHSS), simple Fugl-Meyer score (FM), Barthel Index (BI), Montreal Cognitive Assessment (MOCA) were used to evaluate the neurological deficits, motor function, daily living ability and cognitive function 2 hours before the MRI examination, respectively. The mRs score was used to evaluate the patients' neurological function recovery after 30 days. The plasma BDNF concentration was employed by enzyme linked immunoassay technique (ELISA). Three dynamic indicators of rs-fMRI, dALFF, dfALLFF, and dReHo, were calculated for all subjects. Brain differences between the patients with CS and healthy controls were evaluated by DPABI V5.1, and the significance level was set at a voxel level of p<0.05 and a cluster level of p<0.05, two tailed, with GFR correction. Spearman correlations were analyzed between the three indicators and clinical outcome indicators in CS group. p<0.05 was considered statistically significant.Result
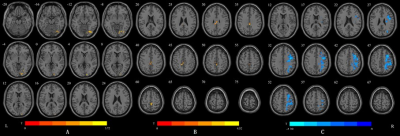
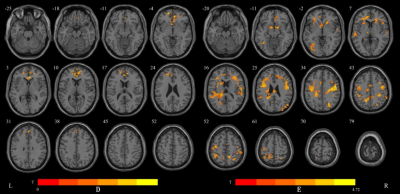
1) Compared with controls, CS group had significantly increased dALFF values in the right lingual gyrus, the right fusiform gyrus and the right inferior occipital gyrus, and significantly increased dfALFF values in the middle cingulum gyrus and the right paracentral lobule. However, significantly decreased dReHo values were found in the right precentral gyrus, the right middle frontal gyrus, the right postcentral gyrus and the right inferior parietal gyrus in the CS group (voxel p<0.05,cluster p<0.05,two tailed) (Figure1)2)Compared with D7 patients, dALFF values of D30 patients significantly increased in the medial superior frontal gyrus and bilateral anterior cingulate. But in the dfALFF analysis, no brain area was changed. The dReHo values of bilateral precentral gyrus, the left inferior parietal gyrus and the left superior superior temporal gyrus were significantly increased (voxel p<0.05,cluster p<0.05,two tailed) (Figure 2).
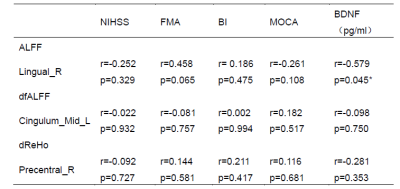
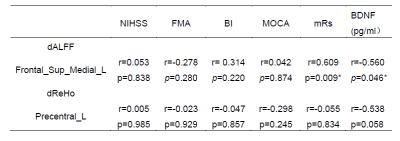
3)There were negative correlations between the plasma BDNF concentration and dALFF values in right lingual gyrus compared with controls (rs=0.-579, P=0.045) (Table1) and in left medial superior frontal gyrus (rs=-0.560, P=0.046) in different examination time(Table2) . Besides, the dALFF value in left medial superior frontal gyrus was positively correlated with the motor function in Day 30 (rs=0.609, P=0.009). In addition, changes in plasma BDNF concentration between D7 and D30 were positively correlated with MOCA score (rs=0.612, P=0.026) (Table2).
Discussion and Conclusions
At 30 days after infarct onset, dALFF value of the left medial superior frontal gyrus had negative correlations with changes of plasma BDNF concentration and positive correlations with mRs score, suggesting that the brain area around the lesion after stroke has, to some degree, neurological remodeling. Therefore, the superior frontal gyrus may be used as a potential therapeutic target to promote the recovery rate of movement in patients with CS. In addition to the correlation between the traditional SMN and motor recovery, such as the angular gyrus overlapping with parts of the default network, we speculate the recovery of motor function of stroke patients is also accompanied by the recovery of cognitive function. Besides, the change of plasma BDNF is positively correlated with MOCA score, which also confirms the correlation between plasma BDNF and cognitive function, which was consistent with the results of previous studies4. Furthermore, we found the plasma BDNF concentration is correlated with changes in local brain activity whether in comparison with HCs or longitudinal groups. This aspect may reflect that the brain with subacute CS is in a dysfunctional state, leading to a decrease in plasma BDNF. Low plasma BDNF may be associated with increased risk of stroke5.Acknowledgements
noReferences
1.Stinear CM, Byblow WD, Ackerley SJ, et al. Proportional MotorRecovery After Stroke: Implications for Trial Design[J]. Stroke, 2017, 48(3): 795-798.
2.Balkaya M and Cho S. Genetics of stroke recovery: BDNF val66metpolymorphism in stroke recovery and its interaction with aging[J].Neurobiol Dis, 2019, 126: 36-46.
3.Ploughman M, Windle V, MacLellan CL, et al. Brain-derived neurotrophic factor contributes to recovery of skilled reaching after focal ischemia in rats[J]. Stroke, 2009, 40(4): 1490-1495.
4.Choi SH, Bylykbashi E, Chatila ZK, et al. Combined adultneurogenesis and BDNF mimic exercise effects on cognition in an Alzheimer's mouse model[J]. Science, 2018, 361(6406).
5.Chaturvedi P, Singh AK, Tiwari V, et al. Brain-derived neurotrophicfactor levels in acute stroke and its clinical implications[J]. Brain Circ,2020, 6(3): 185-190.
Figures