4465
A study on structural changes of limbic systems in cerebral small vascular disease patients with mild to moderate depression
Kun Li1, Dongtao Liu2, Xiuqin Jia1, Qiao Bu1, Rui Jia1, Tao Jiang1, Yueluan Jiang3, Qinglei Shi3, Zhenyu Pan1, and Lichun Zhou2
1Department of Radiology, Beijing Chao Yang Hospital, Beijing, China, 2Department of Neurology, Beijing Chao Yang Hospital, Beijing, China, 3MR Scientific Marketing, Siemens Healthineers, Beijing, China
1Department of Radiology, Beijing Chao Yang Hospital, Beijing, China, 2Department of Neurology, Beijing Chao Yang Hospital, Beijing, China, 3MR Scientific Marketing, Siemens Healthineers, Beijing, China
Synopsis
Major depressive disorder severity is associated with limbic systems grey matter volumetric reductions, while for cerebral small vascular disease (CSVD) patients with mild to moderate depression the pathological mechanism needs to be future investigated. Diffusion kurtosis imaging (DKI) is an advanced diffusion model that characterizes water diffusion process as non-Gaussian distribution. DKI parameters are highly sensitive to the micro-environment of tissues, especially for the anisotropic structure. This study aimed to investigate the structure changes of limbic systems in patients with cerebral small vascular disease (CSVD) induced mild to moderate depression by applying diffusion kurtosis imaging.
Introduction
Most elderly people have neuroimaging evidence of cerebral small vessel disease (CSVD), which is a common disease of small perforating arteries and capillaries. CSVD leads to cerebral microcirculation disorder and mainly affects deep white matter and gray matter [1]. The high burden of CSVD is associated with mood disorders, including cognitive and depression in late life [2]. The pathobiology of depression in CVSD patients is incompletely understood. Current research shows that major depressive disorder severity is associated with limbic systems grey matter volumetric reductions with worsening depressive symptoms [3]. While the changes in gray matter volume and pathological mechanism in CVSD patients with mild or moderate depression remain unclear. Diffusion kurtosis imaging (DKI) is an advanced diffusion model based on multiple b values. It describes the diffusion process of water as a non-Gaussian distribution. Therefore, the parameters derived from DKI are very sensitive to the changes of microstructure and the compatibility of anisotropic environment[4]. Limbic systems is an important structure involved in memory processing and emotional management. This study aimed to investigate the limbic systems volume change and microstructures alteration in CSVD patients with mild to moderate depression by applying diffusion Kurtosis imaging.Method
71 CSVD patients (median age: 64 (60,69) years, 41 males, 30 females) were enrolled in this study. All of them were assessed by Hamilton depression rating scale (HAMD). The subjects were divided into mild to moderate depression group (CSVD-D, n=39, HAMD score 8-23 points) and non-depression group(CSVD-ND, n=32, HAMD score < 7 points. All patients underwent MRI scan on a 3T MR scanner (MAGNETOM Skyra, Siemens Healthcare, Erlangen, Germany). T1 weighted images (T1WI) were captured by three-dimensional magnetization prepared rapid acquisition gradient echo (T1WI 3D MPRAGE) for bilateral amygdala, anterior cingulum cortex (ACC), and hippocampus volume measurement, which is calculated based on the Anatomical Automatic Labeling (AAL) template by an in-house developed software Brainquan tool [5]. The diffusion imaging based on spin-echo echo planar imaging (SE-EPI) was obtained along 30 directions, with 3 b values (0, 1000, 2000 mm2/s). The sequence parameters were as follows: TR/TE= 7700/89ms, iPAT= 2, FOV= 222 × 222mm, matrix= 74 × 74; slice thickness:3mm; number of slices= 50; acquisition time 588s. The parameters derived from DKI were calculated by DKE software (http://www.nitrc.org/projects/dke, version 2.5.1). The DKI parameters include kurtosis fractional anisotropy (KFA), mean kurtosis (Kmean) and mean diffusivity (Dmean). T1WI 3D MPRAGE and DKI maps were registered into MNI space, and the amygdala, ACC, and hippocampus were automatically segmented by applying SPM8 (http://www.fil.ion.ucl.ac.uk/spm) and the mean value of the parameters were calculated based on the Anatomical Automatic Labeling (AAL) template. The DKI parameters were calculated in regions of bilateral amygdala, ACC, and hippocampus. The volume difference between groups was evaluated by unpaired t test. The intergroup differences of DKI parameters were evaluated appropriately with Student’s t test, the Mann–Whitney U-test or χ2 -tests. Spearman rank correlation analysis was used to determine the association between pairs of variables for non-parametric data. P values below0.05 (two-tailed) were considered statistically significant.Results
The statistic results were summarized in Table 1 and Table2. Compared with CSVD-ND group, CSVD-D group patients showed significantly higher Dmean in both bilateral ACC and amygdala, and left hippocampus. And there are significantly lower KFA of Left ACC and bilateral amygdala in CSVD-D group compared with CSVD-ND group. Furthermore, there is a positive correlation between parameter Dmean and HAMD score in left ACC and bilateral amygdala (The left ACC: R2= 0.126; The left amygdala: R2= 0.097; The right amygdala: R2= 0.079, respectively) and there was a negative correlation between KFA and HAMD score in bilateral amygdala (The left amygdala: R2= 0.072; The right amygdala: R2= 0.066, respectively) as shown in figure 2. No significantly difference were found between CSVD-ND group and CSVD-D group in amygdala volume.Discussion and Conclusion
In this study, we studied the microstructure changes and volume changes of amygdala, ACC and hippocampus in patients with CSVD-induced mild to moderate depression. The results showed that the Dmean and KFA in DKI maps might be early changes of CSVD-induced depression. While the volume might not be changed in CSVD-induced mild to moderate depression. The DKI parameters of ACC and amygdala are potentially useful parameters for the quantitative evaluation of CSVD-induced mild to moderate depression.Acknowledgements
No acknowledgement found.References
1. Wardlaw JM, Smith C, Dichgans M. Mechanisms of sporadic cerebral small vessel disease: insights from neuroimaging[J]. The Lancet. Neurology, 2013, 12(5): 483-497. 2. Taylor WD, Aizenstein HJ, Alexopoulos GS. The vascular depression hypothesis: mechanisms linking vascular disease with depression[J]. Mol Psychiatry, 2013, 18(9): 963-974. 3. Brown, S S G et al. “Structural MRI at 7T reveals amygdala nuclei and hippocampal subfield volumetric association with Major Depressive Disorder symptom severity.” Scientific reports vol. 9,1 10166. 15 Jul. 2019, doi:10.1038/s41598-019-46687-7 4. Lätt J, Nilsson M, Wirestam R, et al. Regional values of diffusional kurtosis estimates in the healthy brain[J]. Journal of magnetic resonance imaging: JMRI, 2013, 37(3): 610-618. 5. F. Xiang Feng, L.X. Meng, Y. Guang, BrainQuan: an integrated tool for automated and region-specific analysis of multi-parametric brain MRI data, Proceedings of the27th Annual Meeting of ISMRM, Montreal (2019) abstract 1591.Figures
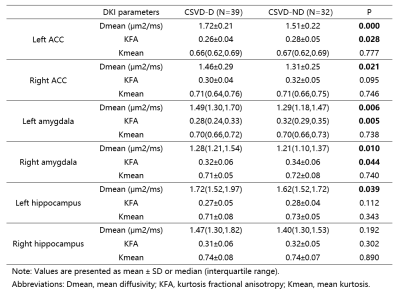
Table
1. A comparison of DKI derived
parameters in amygdala, ACC, and hippocampus between two groups.
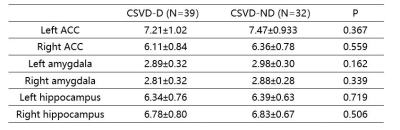
Table 2. A comparison of amygdala,
ACC, and hippocampus volumes between two groups.
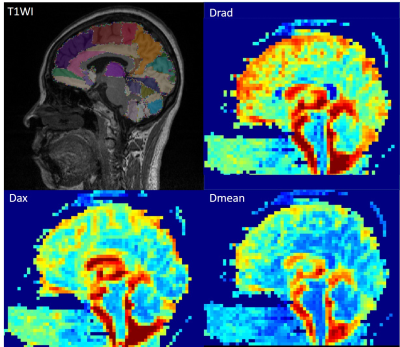
Figure 1. T1WI and DKI maps of a CVSD-ND patient. T1WI 3D is acquired by MPRAGE, sagittal
image, with AAL template segmented different structures.

Figure
2. The correlation
between the DKI parameters of the bilateral
amygdala
and
left ACC
with
HAMD score. Abbreviations: Dmean_cingulum_Ant_L, the mean
diffusivity of
the left anterior
cingulum cortex; Dmean_Amygdala_L/R,
the mean diffusivity of the left/right amygdala; KFA_Amygdala_L/R,
kurtosis fractional anisotropy
of
the left/right amygdala; Hamilton
Depression rating scale.
DOI: https://doi.org/10.58530/2022/4465