4456
Average Saturation Efficiency Filter (ASEF) for CEST Imaging1University of Pittsburgh, Pittsburgh, PA, United States
Synopsis
Endogenous CEST signal usually has low specificity due to contamination from the magnetization transfer effect and from fast exchanging labile protons with close Larmor frequencies. We propose to improve CEST signal specificity with an ASEF which measures the difference between CEST signals acquired with similar average saturation power but largely different duty cycles (DC), e.g., a continuous wave or a high DC pulse train versus a low DC one. Simulation and creatine phantom studies showed that ASEF can improve the specificity of slow to intermediate exchanging CEST signal with a relatively small loss of sensitivity.
Introduction
CEST MRI signal is usually contaminated by magnetization transfer (MT) from semi-solids and overlapping faster, broad exchanges. Numerous solutions have been proposed to remove confounding effects but these methods often either increase acquisition time or sacrifice sensitivity1-4. We propose an Average Saturation Efficiency Filter (ASEF) which uses two pulse trains with similar average saturation power but highly unequal duty cycles, so that saturation transfer effects from fast chemical exchange species and semisolid macromolecules are similar between the two pulse trains, but drastically different for slow exchange species. Thus, their difference becomes an exchange rate filter suppressing MT and fast exchange signals. In this work, the signal properties of ASEF were evaluated by computer simulation and validated by phantom experiments.Methods
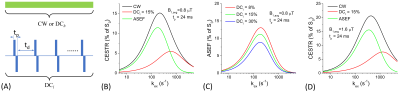
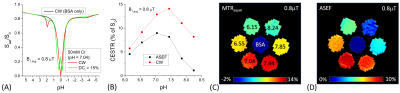
Simulations: Assuming a train of block pulses where the RF in each repeating unit has a bipolar pulse (which minimizes the rotation effect 2) with duration tp (Fig. 1). ASEF takes the difference between CEST signals measured by a continuous wave (or high duty cycle) saturation and a low duty cycle (DCl) one. CEST signals were simulated by Bloch-McConnel Equations which include 3 exchanging pools of free water protons, labile protons, and bound water protons, assuming a chemical shift between the labile proton and water of 1.9 ppm, a fraction of labile proton of 0.001, the T1 (T2) of water, labile proton, and bound water protons is 2 s (66.6 ms), 2 s (66.6 ms), and 2 s (10 ms), respectively.Phantom experiments: MR experiments were performed at 9.4 T. Seven phantoms were prepared in 10% Bovine Serum Albumin (BSA). 50 mM Creatine was added to six of them and they were then titrated to pH = 6.15, 6.55, 7.04, 7.44, 7.85, 8.25, and 7.0 for the BSA only phantom. These phantoms were heated to 95ºC to denature the BSA and measured at room temperature. The saturation preparation schemes consisted of either a single CW block pulse or a DCl = 15% and tp = 24 ms bipolar pulse train, with a duration of 6-s. The B1 was 0.8 μT for the CW and a nominal average B1 = 0.82 μT was determined for the DCl pulse train. This 2.4% fudge factor was obtained by matching the saturated signal at 6 ppm (where the CEST effect is minimal) to minimize the mismatch due to our RF power linearity/stability and the mismatch in the MT effect expected from two saturations with highly unequal duty cycles 5. Images were acquired by single slice spin-echo EPI.
Results
Fig. 1B shows simulated exchange rate filtering of ASEF. The CEST contrast of the DCl pulse train is like that of CW at fast exchange rates but is much smaller at slower exchange rates. As a result, the sensitivity of their difference, i.e., the ASEF signal, is only slightly lower than CW for slow exchange rates but is suppressed at kex > 2000 s-1. For larger DCl values, the sensitivity of ASEF decreases but the exchange rate filtering (e.g., peak position and the linewidth) remains the same (Fig. 1C). With a higher B1, avg = 1.6 µT, the ASEF signal peak shifts to a higher kex, and CEST contrast is suppressed for kex > 4000 s-1 (Fig. 1D). For creatine in heated-denatured BSA, the Z-spectra of the pH = 7.04 phantom show larger differences in the 1.9 ppm dips for CW than DCl pulse train saturation (Fig.2A). The spectra match well for offsets > 3.5 ppm indicating the MT effect can be effectively minimized by ASEF. In Fig. 2B, the CEST contrast of CW saturation is calculated by subtracting the signals of phantoms with creatine from that of BSA only. The ASEF signal is only slightly lower than that of CW at lower pH values, but the difference is much larger at higher pH. Note MTRasym showed a negative baseline of about -2% due to the intrinsic asymmetry of the MT effect, as shown in the BSA-only phantom (Fig. 2C). In contrast, the ASEF signal is minimal for the phantom of BSA only and is small for pH = 8.24 (Fig. 2D), indicating that signals from both the MT contrast and from fast chemical exchange can be effectively suppressed.Discussions
ASEF can improve the CEST signal specificity for slow to intermediate exchanges (e.g., kex <2000 s-1) with only a small reduction in the sensitivity. It can be acquired at as few as only one frequency, i.e., the Larmor frequency of the labile proton of interest. ASEF has three parameters that can be adjusted: the B1, avg determines the range of exchange rate filtering, the duty cycles of the pulse trains determine ASEF sensitivity, and tp which is sensitive to direct water saturation. The MT mismatch5 between the low and high DC saturations may be minimized by careful selection of these parameters, as well as a fudge factor of the B1, avg between the two saturations.Conclusion
ASEF is a simple method that can minimize the MT effect and provide fast exchange rate filtering for CEST MRI with a relatively small reduction in sensitivity. It can be a highly useful tool for CEST study in the slow to intermediate exchange regime.Acknowledgements
This work is supported by NIH grant NS100703.References
1. Zaiss M, et al. J Magn Reson 2011;211(2):149-55.
2. Zu Z, et al. Magn Reson Med 2013;69(3):637-647.
3. Xu X, et al. Magn Reson Med 2016;75(1):88-96.
4. Chen L, et al. Magn Reson Med 2019;81(1):69-78.
5. Varma G, et al. J Magn Reson 2018;296:60-71.