4452
Simultaneously Assessing Cerebral Glucose and Oxygen Metabolism and Blood Flow in Rat Brain at Ultrahigh Field of 16.4T1University of Minnesota, Minneapolis, MN, United States
Synopsis
In this study, we used a newly developed tri-frequency RF surface coil that enables 1H MRI and interleaved 2H/17O MRS measurements to simultaneously assess cerebral glucose and oxygen metabolism, cerebral blood flow and oxygen extraction fraction in rat brain at 16.4T with administration of 2H-labeled glucose and 17O-labeled oxygen gas. Our results demonstrate that this approach can capture the activities of major pathways relevant to glucose and oxygen metabolism and perfusion in the same brain at the same time, thereby provide comprehensive information crucial for understanding the metabolic and vascular coupling or uncoupling relationship in healthy and diseased brains.
Introduction
In vivo X-nuclear (e.g., 2H, 13C, 17O and 31P etc.) MRS-based metabolic imaging technologies are promising for studying cerebral metabolism and bioenergetics in animal and human brains1-4. Their detection sensitivities and capabilities are significantly improved at high/ultrahigh field5-7. However, these technologies are often applied independently to obtain relevant metabolic information specific to the nuclei since it is difficult to perform interleaved or simultaneous multi-nuclei measurement and capture different metabolic information or dynamics in the same brain. In this work, we apply a newly developed singular RF coil with active tuning/matching to perform 1H-MRI and interleaved 2H-17O MRS measurements in rat brains at 16.4T8-9. By combining the measurements with 2H-labeled glucose administration and 17O-labeled oxygen gas inhalation, we are able to simultaneously assess the cerebral glucose and oxygen metabolism and blood flow and detect their differences in individual brains.Methods
Two Sprague Dawley rats were mechanically ventilated under 2% isoflurane anesthesia in a mixture of N2O/O2; animal physiology was monitored and maintained under stable condition during MRI/MRS measurements. D-Glucose-6,6-d2 (Cambridge Isotope Lab; 1g/kg dissolved in 1.8mL saline) were administered intravenously within 2-min after 5-min baseline MRS acquisition; two consecutive 17O2 (74.8% enrichment, Sigma-Aldrich, mixed with N2O and isoflurane) gas inhalations (~2min each) per rat were occurred during and 30min-post the D66 administration, respectively. All MR experiments were conducted on 16.4T/26cm scanner (Varian/VNMRJ) using a newly developed 1H/2H/17O surface coil (2.5cm diameter)8-9.After 1H MRI and B0 shimming, 80-min dynamic 17O-2H MRS data of the rat brains were acquired using an interleaved pulse-acquire sequence for 2H (TR=300ms, nt=1, sw=4006Hz, acquisition time (at)=44ms, Ernst angle) and 17O (TR=50ms, nt=5, sw=4006Hz, at=44ms, nominal 90o flip angle). The 2H-17O data were processed with 15Hz line broadening, respectively, before Fourier transformation to enhance spectral signal-to-noise ratio. A Matlab-based program was used to fit all resonance signals and the integrals were used to quantify the metabolites concentration after correcting the saturation effect. The cerebral blood flow (CBF), cerebral metabolic rate of oxygen (CMRO2) were calculated from the corresponding slopes and exponential decay rates of the H217O dynamics1, 10, they determined oxygen extraction fraction (OEF)11.
Results
Table1 lists the body weight and physiological conditions of the rats, where Rat A appears weaker than Rat B. Figure 1 displays the stack plots of the dynamic 2H-17O spectra obtained during the 80-min data acquisition in the two rats underwent simultaneous D66-glucose (IV) administration and 17O2 gas inhalation after 5-min baseline and a subsequent 17O2 gas inhalation 30min-post the D66-glucose injection. The quality of the 2H-17O spectral data and the dynamic changes of relevant metabolites are clearly shown. After careful data analysis, the concentration (mM) time courses of 17O-labeled water (Fig. 2), and 2H-labeled water, glucose, Glx and lactate (Fig. 3) were obtained.The results of CMRO2 and CBF are summarized in Fig. 2, showing higher CBF and lower CMRO2 in Rat A compared to Rat B. Interestingly, we detected a large CBF decrease (>30%) and a smaller CMRO2 reduction (<5%) about 30min post-D66 in both rats, indicating a possible effect of glucose administration. The averaged OEF was 0.28 in Rat A; significantly lower than that of 0.40 in Rat B.
Figure 3 summarizes the dynamics of deuterated metabolites associated with cerebral glucose metabolism, TCA cycle activity and lactate production. Rat B has much higher TCA cycle activity with almost twice of Glx produced as that of Rat A. Additionally, the lactate production in Rat B is slightly slower and less than that of Rat A, consequently, higher HDO content and slower Glc decay were observed in Rat B.
Figure 4A displays the calculated Lac/Glx ratio and its dynamics in the two rat brains. The higher Lac/Glx ratios seen in Rat A is mainly due to its lower Glx content, rather than a combined effect of higher Lac and lower Glx production as observed in brain tumor, which showed much higher Lac/Glx ratio (Fig. 4B)12-13.
Discussion and Conclusion
To overcome the technique challenges for simultaneously studying cerebral glucose and oxygen metabolism and perfusion using combined 2H-17O MRS technologies, we utilized a novel tri-frequency surface coil with modified pulse sequence and hardware allowing active switching between the 2H and 17O frequencies. The capability and sensitivity of this technology in providing critical and comprehensive metabolic-physiological information of the individual brain in the same measurement are demonstrated. This approach not only saves time, but also improves the measurement accuracy by minimizing variations associated with animal condition changes. In this study, we successfully captured the metabolic/vascular differences of the two rat brains associated with their brain states. Furthermore, the observed differences of cerebral glucose metabolism, oxygen consumption, and TCA cycle activity or lactate production in the same brain and/or between the two brains are consistent with each other.The relatively low CMRO2 values observed in this study is due to the partial volume effect cause by large coil size relative to the rat brain. Adding special localization or imaging capability would further improve the utility and potential of this technique for broad biomedical applications (e.g., stroke, tumor and neurodegenerative diseases).
Acknowledgements
This work was supported in part by NIH grants of R01 MH111413, R01 CA240953, R01 NS118330, U01 EB026978, S10 RR025031, P41 EB027061 and P30 NS076408.
References
1. Zhu XH et al. In vivo 17O NMR approaches for brain study at high field. NMR in Biomedicine 18, 83-103, doi:10.1002/nbm.930 (2005).
2. Lu M et al. Quantitative assessment of brain glucose metabolic rates using in vivo deuterium magnetic resonance spectroscopy. J Cereb Blood Flow Metab 37:3518-3530 (2017).
3. De Feyter HM et al. Deuterium metabolic imaging (DMI) for MRI-based 3D mapping of metabolism in vivo. Sci Adv 4, eaat7314 (2018).
4. Zhu XH, Lu M & Chen W. Quantitative imaging of brain energy metabolisms and neuroenergetics using in vivo X-nuclear 2H, 17O and 31P MRS at ultra-high field. Journal of Magnetic Resonance 292, 155-170 (2018).
5. Lu M et al. In vitro and in vivo studies of 17O NMR sensitivity at 9.4 and 16.4 T. Magn Reson Med 69(6):1523-7 (2013).
6. Lu M, Chen W, Zhu XH. Field dependence study of in vivo brain 31P MRS up to 16.4 T. NMR Biomed 27(9):1135-41 (2014).
7. de Graaf RA et al. On the magnetic field dependence of deuterium metabolic imaging. NMR in Biomedicine 33:e4235 (2020).
8. Zhang GL et al. A dual-frequency surface coil design comprised of a single loop for both proton and deuterium magnetic resonance imaging at 16.4T. Proceedings of ISMRM 28: 4105 (2020).
9. Jenkins PJB et al. Single loop tri-frequency surface coil design for 1H MRI and interleaved dynamic 2H and 17O MRS applications at ultrahigh field of 16.4T. Proceedings of ISMRM 29: p. 1816 (2021).
10. Zhu XH et al. In vivo measurement of CBF using 17O NMR signal of metabolically produced H217O as a perfusion tracer. Magn Reson Med 70(2):309-14 (2013).
11. Zhu XH et al. Simultaneous and noninvasive imaging of cerebral oxygen metabolic rate, blood flow and oxygen extraction fraction in stroke mice. Neuroimage 64, 437-447 (2013).
12. Zhu XH et al. Dynamic deuterium MRS imaging of brain tumor with enhanced sensitivity and spatio-temporal resolution. Proceedings of ISMRM 28: p. 271 (2020).
13. Li Y et al. Machine Learning-Enabled High-Resolution Dynamic Deuterium MR Spectroscopic Imaging. IEEE Trans Med Imaging doi:10.1109/TMI.2021.3101149 (2021).
Figures

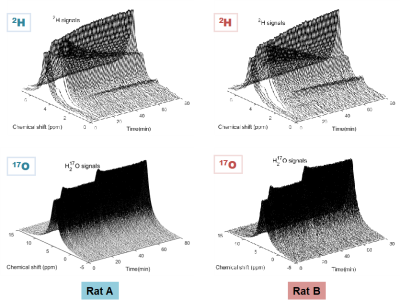
Figure 1. Stack plots of the dynamic 2H and 17O brain spectra obtained during 80-min continues acquisition in the two rats underwent simultaneous administration of D66-glucose (IV) and 17O2 gas inhalation from 5 to 7 min, and a subsequent 17O2 gas inhalation occurred around 35-37 min.
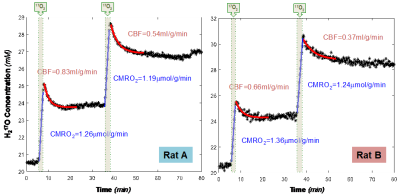
Figure 2. The time courses of the H217O water concentration and the corresponding CBF and CMRO2 values of the two rats during the two repeated 17O2 inhalations. We observed relatively large CBF and small CMRO2 differences between the two rats, while the CBF decreased more than 30% in half hour after the D66 administration in both rats.
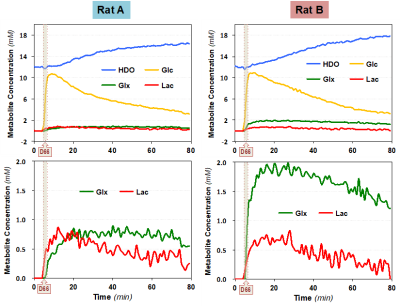
Figure 3. The time courses of deuterated water (HDO), glucose (Glc), glutamate/glutamine (Glx) and lactate (Lac) concentrations of the two rats before, during and after the D66 administration are displayed. We detected large difference in the Glx production between the two rat brains, while smaller differences in HDO accumulation, Glc consumption/washout and Lac production were also observed.
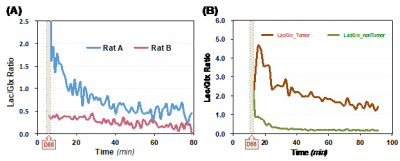
Figure 4. (A) The brain Lac/Glx ratios after the D66 administration are calculated and presented, which are substantially different between the two rats studied. The increased Lac/Glx ratio observed in Rat A is still much lower than that of brain tumor (B) observed in a previous study12.