4449
Quantitative Susceptibility Mapping for Chamber Blood Oxygenation in Pulmonary Hypertension: Validation using Right Heart Catheterization1Biomedical Engineering, Cornell University, New York, NY, United States, 2Radiology, Weill Cornell Medicine, New York, NY, United States, 3Medicine, Weill Cornell Medicine, New York, NY, United States
Synopsis
Pulmonary hypertension (PH) is a progressive and life shortening disorder with increased differential blood oxygen saturation (ΔSaO2) between right and left heart. In this prospective study, we acquired cardiac QSM from patients undergoing clinically indicated right heart catheterization (RHC) for assessment of known or suspected PH. ΔSaO2 estimated from QSM aligned well with RHC oxygenation data, showing QSM as a non-invasive CMR technique can be applied to clinically evaluate heart chamber oxygenation quantification in PH.
Introduction
Pulmonary hypertension (PH) is a progressive and life shortening disorder that is growing in prevalence and affects up to 10% of adults over the age of 65, with substantial risk of dyspnea, reduced effort tolerance and mortality. Impaired lung oxygenation and increased differential blood oxygen saturation (ΔSaO2) between right and left heart are key parameters in PH, which have been used to guide therapeutic decision-making and clinical prognosis. Invasive cardiac catheterization is the current clinical standard for measuring cardiovascular blood oxygenation. Non-invasive cardiac MRI (CMR) is widely used to assess cardiac structure and function in patients with PH but currently lacks a robust and clinically feasible way of measuring cardiac chamber oxygenation. Recently, cardiac quantitative susceptibility mapping (QSM) has been proposed to directly measure ΔSaO2 in clinical settings[1]. In this study, this technique is performed in a cohort of pulmonary hypertension patients and compared with right heart catheterization.Methods
In this prospective study, 14 patients underwent clinically indicated right heart catheterization (RHC) for assessment of known or suspected pulmonary hypertension, as well as cardiac MRI on a GE 3T clinical scanner. As part of their cardiac MRI, QSM was performed 20-30 minutes post gadolinium administration (0.2mmol/kg) in either short-axis or axial view. All subjects provided consent for this IRB approved protocol. The data were acquired using a free-breathing ECG-triggered 3D multi-echo gradient echo sequence[1] with 4mm 1D diaphragmatic navigator (typical spatial resolution 1.5×1.5×5mm3, under-sampling ratio =2). Two navigators were acquired pre and post data acquisition in each segment to limit respiratory motion using phase ordering with automatic window selection (PAWS). To reconstruct QSM, the multi-echo complex data were first fitted to get the initial total field and unwrapped by graph-cut based method. Iterative decomposition of water and fat with echo asymmetry and least squares estimation (IDEAL) was used for water-fat separation. Then total field inversion (TFI+0) together with regularization of blood pool susceptibility variation was performed[2-4]:$$
y^* = \underset{y}{\mathrm{argmin}}||w(f-DPy)||_2^2 + \lambda ||M_G\triangledown Py||_1 + \sum_i \lambda_i||M_iP(y-\overline{y}^i)||^2_2
$$
The first and second terms are in accordance with classical MEDI reconstruction representing the data consistency and L-1 penalty; the last one imposes additional uniformity regularization on the heart chamber blood pools regions, where index $$$i$$$ represents for the left/right ventricle regions segmented from the combined-echo magnitude; $$$P$$$ is the preconditioner term to improve the algorithm convergence. The final QSM is computed as $$$\chi = Py^*$$$.
To quantify differential oxygen saturation between the left and right heart, the mean susceptibility values from the regions of left/right ventricle blood pools were derived and their difference was scaled based on each individual hematocrit (Hct) to estimate ΔSaO2. The results were compared with RHC reported values to validate the QSM quantification. Deming regression and Bland Altman plots on the two measurements were conducted. Image post-processing was performed using MATLAB 2021a. Statistical analysis was performed using R 4.1.2.
Results
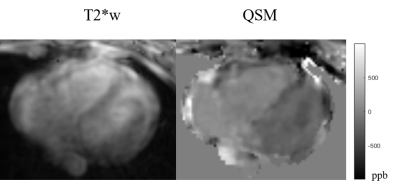
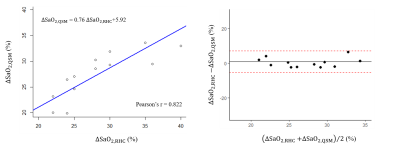
14 patients (age: 54±13yo, 23% male) with PH underwent cardiac QSM (acquisition time 594±283.7sec, navigator efficiency 29%, resolution 1.5x1.5x5mm3). All patients had RHC with reported ΔSaO2 between pulmonary artery and aorta within 2 weeks of their CMR examination (mean interval 6 ± 5days [70% pre / 30% post CMR]. One subject failed in the cardiac QSM due to excessive motion. Five subjects had QSM acquired in axial plane while the remaining were in short axis orientation. Figure 1 shows one representative case of QSM and corresponding T2* weighted magnitude from multi-echo GRE in axial. LV with oxygenated blood demonstrates more negative susceptibility values on QSM compared to RV with deoxygenated blood.Among the 13 patients, the mean difference of susceptibility between LV and RV is 299.32 ± 115.97ppb, resulting in the estimated mean differential oxygen saturation between LV/RV 25.08 ± 6.90%, corresponding to their reported ΔSaO2 29.07 ± 6.02%. Method comparison analysis (Figure 2) shows good linear relationship between the two measurement ΔSaO2,QSM = 0.76 ΔSaO2,RHC + 5.92 (%), suggesting the ability of QSM to quantify heart chamber oxygenation difference.
Discussion
In this PH cohort, cardiac QSM was successfully acquired in all but one patient. However, the scan efficiency is limited due to prospective navigator acquisition. The QSM enables non-invasive measurement of oxygen saturation difference among heart chambers, validated by RHC ΔSaO2. Currently, QSM reconstruction and oxygen quantification demand heart chamber segmentation. An automated pipeline of image segmentation and optimization will allow the inline reconstruction in the future.Conclusion
Cardiac QSM provides non-invasive measurement of heart chamber oxygen saturation difference via validation of RHC in this preliminary PH patient study.Acknowledgements
The authors acknowledge the National Institutes of Health for grant support.References
1. Wen, Yan, et al. "Free breathing three-dimensional cardiac quantitative susceptibility mapping for differential cardiac chamber blood oxygenation–initial validation in patients with cardiovascular disease inclusive of direct comparison to invasive catheterization." Journal of Cardiovascular Magnetic Resonance 21.1 (2019): 1-13.
2. Liu, Zhe, et al. "Preconditioned total field inversion (TFI) method for quantitative susceptibility mapping." Magnetic resonance in medicine 78.1 (2017): 303-315.
3. Liu, Zhe, et al. "MEDI+ 0: morphology enabled dipole inversion with automatic uniform cerebrospinal fluid zero reference for quantitative susceptibility mapping." Magnetic resonance in medicine 79.5 (2018): 2795-2803.
4. Wen, Yan, et al. "Cardiac quantitative susceptibility mapping (QSM) for heart chamber oxygenation." Magnetic resonance in medicine 79.3 (2018): 1545-1552.
Figures