4442
Early Detection of Radiation-Induced Cardiotoxicity Using Hyperpolarized Pyruvate1Advanced Imaging Research Center, UT Southwestern Medical Center, Dallas, TX, United States, 2Radiation Oncology, UT Southwestern Medical Center, Dallas, TX, United States, 3Internal Medicine, UT Southwestern Medical Center, Dallas, TX, United States, 4Molecular Biology, UT Southwestern Medical Center, Dallas, TX, United States, 5Radiology, UT Southwestern Medical Center, Dallas, TX, United States, 6Electrical and Computer Engineering, UT Dallas, Richardson, TX, United States
Synopsis
Radiation-induced heart disease is a major source of morbidity and mortality in patients receiving thoracic radiation. In this study, radiation-induced changes in cardiac metabolism is investigated using hyperpolarized [1-13C]pyruvate MRI in animals and patients. Myocardial bicarbonate-to-lactate ratios decreased following radiation treatments while no change was observed in the global strain, suggesting radiation-induced mitochondrial dysfunction in the heart. This translational study demonstrates clinical potential of hyperpolarized 13C pyruvate for early and noninvasive detection of radiation-induced cardiac injury.
Background
Radiation therapy is a routine treatment option for patients with intrathoracic malignancies. However, patients receiving thoracic radiation have potential risks of developing Radiation-Induced Heart Disease (RIHD), a major source of morbidity and mortality. One of the leading causes of RIHD is mitochondrial dysfunction in the heart.1 Previous animal studies uesed MRI regional strain as a sensitive biomarker for the early detection of radiation-induced cardiac dysfunction.2 Metabolically, hyperpolarized 13C MRI with [1-13C]pyruvate has the potential to detect the mitochondrial dysfunction caused by RIHD. Previous human studies showed that cardiac mitochondrial function can be detected using the hyperpolarized 13C MRI.3,4 In this study, we investigated the feasibility of using hyperpolarized 13C MRI as a noninvasive approach for the early detection of RIHD.Methods
A GE 3T 750w wide-bore scanner and a SPINlab™ DNP polarizer (GE Healthcare) were used for both animal and human studies. For animal studies, eight Sprague-Dawley rats were divided into two groups: the control group (n = 4) and the cardiac radiation group (n = 4). Rats in the radiation group were treated with image-guided cardiac radiation using cone-beam CT (X-Rad 225Cx; a total dose of 40 Gy in 5 fractions). Each rat was imaged three times longitudinally with hyperpolarized [1-13C]pyruvate MRI (before the treatment, one week and six months after the treatment). 13C imaging was performed using a 13C/1H dual-tuned birdcage RF coil and a metabolite-selective spiral imaging sequence5 (FOV = 80 × 80 mm2, spatial resolution = 8 × 8 mm2, slice thickness = 25 mm, TE/TR = 15 ms/213 ms, flip angles = 90° for products and 5° for pyruvate, #timepoints = 16, injection-to-scan time = 15 s) immediately following a bolus injection of 70-mM hyperpolarized [1-13C]pyruvate. Bicarbonate-to-lactate ratios were quantified in the heart. Concurrently, all animals underwent the baseline and post-radiation echocardiography to measure global longitudinal strain. Unpaired t-tests (α = 0.05, two-tailed) were used to compare global strains and bicarbonate-to-lactate ratios between the control and radiation groups.For the patient scan, hyperpolarized 13C imaging was conducted on the vertical long-axis plane of the heart before and after the radiation treatment. A two-loop 13C transmit-receive coil (diameter = 20 cm; PulseTeq Ltd.) and the metabolite-selective spiral imaging sequence (FOV = 40 × 40 cm2, spatial resolution = 1 × 1 cm2, slice thickness = 3 cm, #timepoints = 16, injection-to-scan time = 15 s, flip angles = 90° for products and 10° for pyruvate, TR = 2 R-R) were used with an injection of 250-mM hyperpolarized [1-13C]pyruvate (0.40 mL/kg body weight) as previously described.6 The subject was instructed to hold the breath in expiration for ~20 s, followed by shallow breathing to minimize the respiratory motion. Bicarbonate-to-lactate ratios were calculated in the whole myocardium, anterior and posterior myocardium.
Results and Discussion
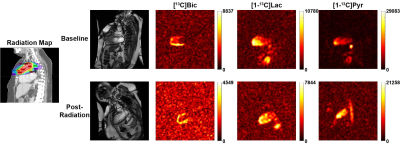
Figure 1 shows time-resolved 13C images of [13C]bicarbonate, [1-13C]lactate and [1-13C]pyruvate from a representative rat at the baseline. No significant differences in the global longitudinal strain were observed between the cardiac radiation and control groups until 2 months post the radiation treatment (p = 0.035), Figure 2. Cardiac bicarbonate-to-lactate ratios measured at one week and six months post the radiation relative to the baseline were quantified for all rats, and normalized by values from the control group at the corresponding timepoint. Significant diversion of longitudinal changes in bicarbonate-to-lactate ratios between the groups were observed at the one-week (p = 0.024) and six-month (p = 0.0002) timepoints (Figure 3). Compared to the control group, the bicarbonate-to-lactate ratio relative to the baseline in the radiation group was 0.677 ± 0.097 at one week post the radiation and 0.396 ± 0.068 at six months post the radiation, respectively. The distinct patterns of longitudinal changes in bicarbonate-to-lactate ratios between the control and radiation groups indicate the change of pyruvate metabolism due to radiation. Especially, the deviation of the pyruvate-to-bicarbonate conversion in the radiation group from the control group is likely due to the mitochondrial dysfunction caused by the radiation treatment, while no change was observed in the global strain.A patient (female, 60 y.o.) with Masaoka-Koga stage III type B2 thymoma was recruited for the study. Between the two hyperpolarized 13C studies, the mediastinum of this patient was treated using intensity-modulated radiation therapy (IMRT) technique, and a dose of 5000 cGy was delivered in 25 fractions of 200 cGy per fraction. Figure 4 shows the time-resolved cardiac hyperpolarized 13C images from the patient on the vertical long-axis plane from the baseline and post-radiation scans. 13C signals from multiple timepoints were added to quantify the myocardial bicarbonate-to-lactate ratios (Figure 5). From this patient, the ratio in the whole myocardium decreased from 0.731 to 0.587 after the treatment. Specifically, bicarbonate-to-lactate ratio in radiated region of the myocardium (anterior) decreased from 0.735 at baseline to 0.669 at post-radiation, while the ratio in the posterior myocardium increased from 0.528 at baseline to 0.569 post-radiation.
Conclusion
The results demonstrate that radiation-induced mitochondrial dysfunction in the heart can be captured by hyperpolarized [1-13C]pyruvate MRI even when the global strain has not shown any changes. The study suggests that hyperpolarized 13C MRI can be potentially used as a noninvasive tool for the early detection of radiation-induced cardiac injury in patients.Acknowledgements
Personnel Support: We appreciate the clinical research team and the supporting staffs of the Advanced Imaging Research Center at UT Southwestern – Crystal E. Harrison, PhD, James Ratnakar, PhD, Jeannie Baxter, RN, Kelley Derner, RN, Salvador Pena, and Maida Tai. We also thank GE Healthcare and Galen Reed, PhD, for the technical support on operating the SPINlab polarizer.
Funding: National Institutes of Health of the United States (P41 EB015908, S10 RR029119, S10 OD018468, R01 NS107409); The Welch Foundation (I-2009-20190330); The Cancer Prevention and Research Institute of Texas (RP180404).
References
1. Livingston K, Schlaak RA, Puckett LL, Bergom C. The role of mitochondrial dysfunction in radiation-induced heart disease: from bench to bedside. Front. Cardiovasc. Med. 2020;7:20.
2. Ibrahim E-SH, Baruah D, Croisille P, et al. Cardiac magnetic resonance for early detection of radiation therapy-induced cardiotoxicity in a small animal model. JACC: CardioOncology. 2021;3(1):113–130.
3. Cunningham CH, Lau JYC, Chen AP, et al. Hyperpolarized 13C metabolic MRI of the human heart: initial experience. Circ Res. 2016;119(11):1177–1182.
4. Park JM, Reed GD, Liticker J, et al. Effect of doxorubicin on myocardial bicarbonate production from pyruvate dehydrogenase in women with breast cancer. Circ Res. 2020;127(12):1568–1570.
5. Ma J, Chen J, Reed GD, et al. Cardiac T2∗ measurement of hyperpolarized 13C metabolites using metabolite-selective multi-echo spiral imaging. Magn Reson Med. 2021;86(3):1494–1504.
6. Ma J, Malloy CR, Pena S, et al. Dual-phase imaging of cardiac metabolism using hyperpolarized pyruvate. Magn Reson Med. 2021;00:1-10.
Figures