4438
Free-breathing Fully Ungated 3D Cardiac T2* MR Mapping using a Low-Rank Tensor Framework1Cedars-Sinai Medical Center, Los Angeles, CA, United States, 2University of California, Los Angeles, Los Angeles, CA, United States, 3University of Southern California, Los Angeles, CA, United States, 4Stanford University, Stanford, CA, United States, 5Indiana University, Indianapolis, IN, United States
Synopsis
A 3D fully ungated, free breathing cardiac T2* technique was developed using a low-rank tensor framework. An animal model with iron-oxide contrast-enhanced studies was used to test and validate the proposed technique. T2* images reconstructed from the proposed approach showed better image quality than those from conventional 2D T2* imaging approach. Excellent agreement of septal T2* was found between the proposed approach and conventional 2D approach. Our findings show that the proposed approach can accurately characterize T2* of myocardium under baseline and during iron overload conditions.
Introduction
T2* cardiac MRI is the gold-standard for assessment of myocardial iron overload such as thalassemia and intramyocardial hemorrhage. However, the conventional breath-held, ECG-gated, multi-gradient-echo T2* images can suffer from motion artifacts from unsuccessful breath holding or irregular heartbeats which is common in cardiac patients. We developed a time-efficient, fully ungated, free breathing, 3D T2* mapping approach using a low-rank tensor framework to address the above issues.Methods
The proposed approach is based on a previously described low-rank tensor framework, where the cardiovascular image A is represented as a 6-dimensional function of 3D spatial location and 3 temporal dimensions which are respiratory motion R, cardiac motion C and T2* decay T. This can be matricized and factored as:$$ A_{(1)}=U_x G_{(1)} (U_R⨂U_C⨂U_T ) ^T $$
G is the core tensor governing the interaction between factor matrices. A is reconstructed in factored form using an explicit tensor subspace constraint:
$$\hat{U}_x=\underset{U_x}{argmin} ‖d-Ω([FSU_x]Φ)‖_2^2+R(U_x ) $$
where $$$Φ$$$ is constructed from the temporal factor matrices as $$$Φ=G_{(1)} (U_R⨂U_C⨂U_T )^T$$$.
An interleaved subset of training data which consists of k-space center lines is acquired to reconstruct motion states of the image tensor by a small-scale LRT completion.
$$\hat{χ}_{tr}=\underset{χ_{tr}}{argmin} ‖d_{tr}-Ω_{tr} (χ_{tr} )‖_2^2+λ\sum_{n = 1}^{4}‖Χ_{tr,(n)} ‖_* +R(χ_{tr}) $$
Once the tensor $$$Φ$$$ is completed, the matrix is extracted from $$$\hat{χ}_{tr}$$$ by higher-order singular value decomposition (HOSVD).
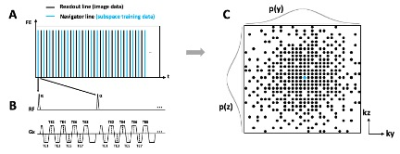
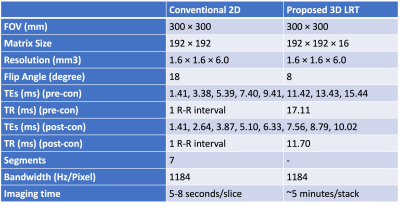
A randomized Gaussian-distributed Cartesian k-space distribution pattern is used to avoid synchronization between sampling and periodic motion (Figure 1). Each readout consists of 8 gradient echoes and K-space center line (shown as blue lines in Figure 1) is collected in an interleaved fashion with every other readout line (shown as black lines in Figure 1).The proposed approach was studied in swines (n = 10, 28 – 32 kg) on a 3.0T MRI system (Verio, Siemens). Conventional breath-hold, ECG triggered 2D multi-gradient-echo, short-axis T2* images of the whole left ventricle were acquired for comparison. Fully ungated, whole heart 3D, LRT data was also acquired under free breathing (scan parameters are shown in Table 1).To evaluate changes in T2* we performed iron-oxide contrast-enhanced studies in a fraction of the animals (n = 5). After baseline scans, an iron-oxide contrast agent was administered to the animal (Ferumoxytol, Feraheme, 4mg/kg, 1:20 dilution, 0.2mg/kg/s IV infusion rate). Conventional 2D T2* images were acquired at mid ventricle 5 minutes post infusion. Subsequently, fully ungated, free breathing, 3D LRT data were acquired. TEs of post-contrast T2* images were adjusted to accommodate for the faster T2* decay in the presence of iron (Table 1).Image quality of native T2*-weighted images was assessed by 2 experienced reviewers based on a 5-point scale (1 (poor) to 5 (excellent)). Short-axis T2* maps were generated from T2*-weighted images with adequate image quality (image score > 2) via pixel-wise least-squares fitting to a mono-exponential. The average T2* of septum (where image artifacts are the least) was measured and recorded for comparison.
Results
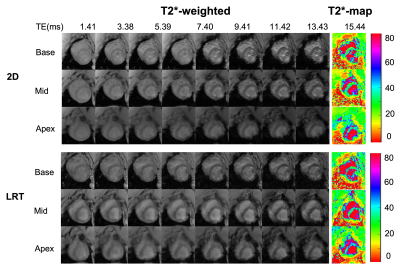
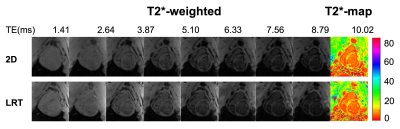
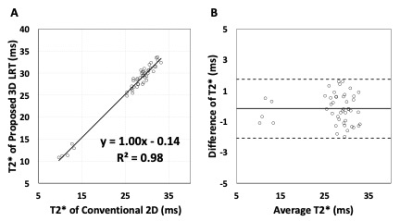
Figure 2 shows native T2* images. T2*-weighted images from conventional 2D eand proposed LRT approaches along with T2* maps of base, mid and apex of left ventricle are shown.Images reconstructed from the proposed LRT approach showed improved image quality than that of conventional 2D approach (image quality score of LRT group was 3.7 ± 0.3 vs. 3.3 ± 0.4 with 2D conventional, p<0.05). There was no difference in T2* values measured from conventional 2D approach and proposed 3D LRT approach in both pre- and post-contrast conditions. At baseline, average T2* of septum from conventional 2D approach is 29.3 ± 2.2 ms and average T2* measured from images in proposed 3D LRT group is 29.1 ± 2.0 ms (p = 0.67). Post-contrast T2* measured from images by conventional 2D approach is 12.0 ± 1.3 ms and 11.6 ± 1.5 ms by the proposed 3D LRT approach (p = 0.76). Examples of post-contrast T2* images from conventional 2D and proposed 3D LRT approaches are shown in Figure 3.Linear regression was performed with both pre- and post-contrast results. T2* between the two approaches showed excellent correlation in Figure 4A (y = 1.00x + 0.14, r2 = 0.98, p<0.05). A Bland-Altman plot with mean of differences and 95% confidence interval is shown in Figure 4B. T2* values from two approaches showed excellent agreement with average bias of less than 1 ms (-0.17 ± 0.96 ms).Discussion
Our results demonstrate that conventional 2D since-slice T2* images, particularly at long TEs, suffer from motion-induced image artifacts (due to imperfect breath-holding or variability in the R-R interval) and, overall, have inferior image quality compared to the proposed 3D LRT approach. Our findings here support the notion that the proposed 3D LRT approach can resolve the cardiac and respiratory motion states to mitigate motion artifacts. Septal T2* values measured from septum showed excellent agreement between the two approaches.Conclusion
We developed, tested and validated a fully ungated, free-breathing 3D cardiac T2* based on low-rank tensor formulation. Our findings here show that the proposed approach can accurately characterize T2* of myocardium under baseline and during iron overload conditions.Acknowledgements
No acknowledgement found.References
1. Christodoulou, A.G., Shaw, J.L., Nguyen, C. et al. Magnetic resonance multitasking for motion-resolved quantitative cardiovascular imaging. Nat Biomed Eng 2, 215–226 (2018).
2. Kellman, P., Xue, H., Spottiswoode, B.S. et al. Free-breathing T2* mapping using respiratory motion corrected averaging. J Cardiovasc Magn Reson 17, 3 (2015).
Figures