4426
Correlation of the 23Na Triple-Quantum Signal with Protein Size1Computer Assisted Clinical Medicine, Heidelberg University, Mannheim, Germany
Synopsis
This study demonstrates a correlation of protein size with sodium triple-quantum (TQ) signal. The TQ/SQ ratio of several globular proteins increased with protein size. Furthermore, strong sodium binding had a substantial impact on the TQ signal. The small protein α-Lactalbumin yielded a strong TQ signal due to strong sodium binding to a calcium binding site using a calcium-depleted protein.
Introduction
Sodium-protein interactions yield a sodium triple-quantum (TQ) signal, a potential biomarker for cell viability. In general, sodium ions only bind weakly to other molecules. Hence, the correlation time $$$\tau_c$$$ is the result of the combined effect of environmental rotational diffusion and the diffusion of the sodium ion itself. Larger proteins tend to rotate slower and therefore the motional regime of sodium ions shifts to the slow motion regime ($$$\omega_0\tau_c\gtrsim1$$$). This results in bi-exponential relaxation and the creation of TQ coherences1. For spherical, globular proteins, the rotational correlation time scales with the molecular weight2,3. A longer correlation time increases the TQ signal and therefore, we propose a TQ signal dependence on the protein size.This study investigated a correlation of sodium TQ signal with protein size using various globular proteins. Most of the used globular proteins either have been shown to be a suitable model protein for TQ signal4 or are well-characterized proteins with knowledge about ion binding sites and affinity for sodium ions5-7.
Materials and Methods
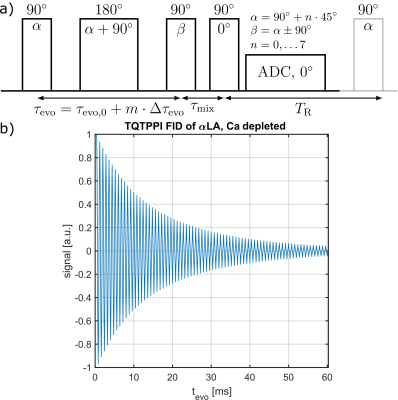
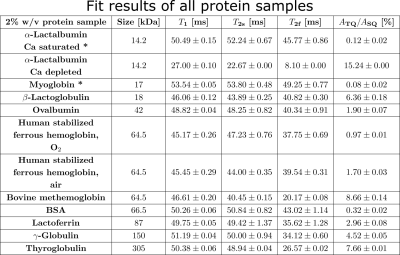
Measurement data was acquired at a 9.4T preclinical MRI (Bruker Biospec 94/20) equipped with either a linear Bruker 1H/23Na volume coil or a quadrature Rapid 1H/23Na volume coil combined with a Rapid 23Na surface coil. Tab.1 lists all used globular proteins. Each samples consisted of 2% w/v protein with 154mM NaCl at pH=7.A TQ time proportional phase incrementation (TQTPPI) pulse sequence8 was used to simultaneously quantify the single-quantum (SQ) and TQ signals (Fig.1a). Simultaneous RF pulse phase and evolution time incrementation enabled a separation and quantification of SQ and TQ signals at distinct frequencies as well as the biexponential $$$T_2$$$ relaxation times. The TQTPPI FID (Fig.1b) was non-linearly fitted using the signal equation8:
$$Y(t)=\sin(\omega t+\phi_1)\cdot\left(A_{SQ,1}e^{-t/T_{2f}}+A_{SQ,2}e^{-t/T_{2s}}\right)+A_{TQ}\sin(3\omega t+\phi_2)\left(e^{-t/T_{2f}}-e^{-t/T_{2s}}\right)+DC $$
where $$$Y(t)$$$ is the TQTPPI FID amplitude, $$$A_{SQ,i}$$$ and $$$A_{TQ}$$$ are the SQ and TQ amplitudes, respectively. $$$T_{2s}$$$ and $$$T_{2f}$$$ are the respective slow and fast transverse relaxation time. TQTPPI sequence parameters were: $$$T_R=5\cdot T_1$$$, 20-60 averages, $$$\Delta\tau_{evo} =100-200\mu s$$$, 600-640 phase steps. Monoexponential $$$T_1$$$ relaxation time was determined by a fit, using an inversion recovery pulse sequence.
Results/Discussion
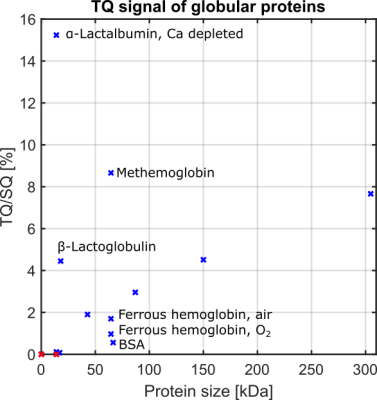
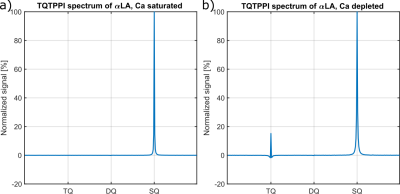
Fig.2 shows the TQ signal for different protein sizes using globular proteins. With the exception of the proteins, Ca depleted α-Lactalbumin (αLA) (14.2kDa), β-lactoglobulin (18kDa), BSA (66.5kDa) and all hemoglobin forms (64.5kDa), the TQ signal increased with protein size. The increased TQ signal indicated a longer correlation time correlating with an increased rotational correlation time of larger proteins. Further increases in protein size result in a rotational correlation time of the protein, which exceeds the diffusional motion of sodium ions. Hence, $$$\tau_c$$$ is now dominated by the diffusional motion and thus, further increases in protein size should have a smaller effect on the TQ signal. This can explain the decreased TQ signal increase for large protein sizes, like thyroglobulin (305kDa) (Fig.2).At small protein sizes, Ca saturated αLA (14.2kDa) and Myoglobin (17kDa) did not yield a TQ signal. This indicated that sodium ions are in the extreme narrowing regime ($$$\omega_0\tau_c\ll 1$$$). Previous studies observed a similar effect in solutions of Ca saturated αLA, amino acids or small molecules such as glycerol and urea despite the use of high concentrations. In contrast, the small proteins beta-Lactoglobulin (18kDa) and Ca depleted αLA (14.2kDa) yielded one of the largest TQ signals. These two proteins are known for their strong sodium binding5-7, which resulted in a slow motional regime for sodium ions due to the dominating slow rotational motion of the protein. In the case of αLA, sodium ions bind to the calcium binding site in the absence of calcium ions. Consequently, the strength of sodium binding has a substantial impact on the TQ signal (Fig.3).
BSA (66.5kDa) and ferrous hemoglobin (64.5kDa) both yielded a weaker TQ signal than the slightly smaller protein Ovalbumin (42kDa) and the slightly larger protein Lactoferrin (87kDa). Carr et al.6 showed that sodium ions do not bind to both proteins at all. However, there is no evidence that binding to both proteins is substantially weaker than to Ovalbumin and Lactoferrin. In contrast, a paramagnetic form of hemoglobin, methemoglobin, yielded an eight times higher TQ signal and, thus, a large TQ signal for this protein size. The large TQ signal in the presence of a paramagnetism, which usually substantially reduces the TQ signal, may indicate an unknown positive effect on the TQ signal.
Excluding the proteins Ca depleted αLA, β-Lactoglobulin, BSA and hemoglobin, due to the aforementioned effects, the measurements indicate that the TQ signal increases with the size of the protein. This can indicate that the in vivo TQ signal originates mainly from larger proteins, which are generally less frequent in the cell9, and sodium binding proteins and. However, further investigations of intracellular proteins and cell lysates are necessary to confirm these results.
Conclusion
The TQ signal of various globular proteins revealed a correlation of TQ signal with protein size and the importance of a strong sodium binding. These results enhance our understanding of the cellular origin of the TQ signal.Acknowledgements
No acknowledgement found.References
1. van der Maarel JRC. Thermal relaxation and coherence dynamics of spin 3/2. I. Static and fluctuating quadrupolar interactions in the multipole basis. Concepts in Magnetic Resonance. 2003;19A(2):97-116.
2. Su X-C, Jergic S, Ozawa K, Burns ND, Dixon NE, Otting G. Measurement of dissociation constants of high-molecular weight protein–protein complexes by transferred 15N-relaxation. J Biomol NMR. 2007;38(1):65-72.
3. Kay LE, Torchia DA, Bax A. Backbone dynamics of proteins as studied by nitrogen-15 inverse detected heteronuclear NMR spectroscopy: application to staphylococcal nuclease. Biochemistry. 1989;28(23):8972-8979.
4. Rooney WD, Springer Jr CS. The molecular environment of intracellular sodium: 23Na NMR relaxation. NMR Biomed. 1991;4(5):227-245.
5. Baker HP, Saroff HA. Binding of Sodium Ions to β-Lactoglobulin*. Biochemistry. 1965;4(8):1670-1677.
6. Carr CW. Studies on the binding of small ions in protein solutions with the use of membrane electrodes. VI. The binding of sodium and potassium ions in solutions of various proteins. Arch Biochem Biophys. 1956;62(2):476-484.
7. Desmet J, Hanssens I, van Cauwelaert F. Comparison of the binding of Na+ and Ca2+ to bovine α-lactalbumin. Biochimica et Biophysica Acta (BBA) - Protein Structure and Molecular Enzymology. 1987;912(2):211-219.
8. Schepkin VD, Neubauer A, Nagel AM, Budinger TF. Comparison of potassium and sodium binding in vivo and in agarose samples using TQTPPI pulse sequence. J Magn Reson. 2017;277:162-168.
9. Brocchieri L, Karlin S. Protein length in eukaryotic and prokaryotic proteomes. Nucleic Acids Res. 2005;33(10):3390-3400.
10. Kleimaier D, Reichert S, Schepkin VD, Schad L. Sodium TQ signal of amino acids and α-lactalbumin in comparison to bovine serum albumin. Proc Intl Soc Mag Reson Med. 2021;29.
Figures