4425
In-tissue nano-assembly of Zn2+-responsive agent provides prolonged imaging time-window for longitudinal in vivo MRI studies1Molecular Chemistry and Material Science, Weizmann Institute of Science, Rehovot, Israel, 2Chemical Research Support, Weizmann institute of Science, Rehovot, Israel
Synopsis
The fast clearance of small molecular probes limits their applicability as responsive agent and prevents their use in longitudinal study. We show here the design and implementation of a tripod-type responsive agent for Zn2+ mapping that assembled to 100nm nanostructure at physiological temperature. The designed fluorinated probe showed capabilities to monitor Zn2+ when combining CEST MRI and 19F-MRI. Upon its intracranial injection the synthetic probe assembled to large entities in the tissue that prevent its clearance from the injection site. This property allows to map its 19F-signal for more than 20 hours and will enable longitudinal studies in the future.
Small molecular imaging probes for MRI applications are important set of sensors that were developed for mapping a variety of analytes ranging from neurotransmitters to metal ions. Nevertheless, their fast clearance from tissues frequently limits their detectability but also prevent the performance of longitudinal studies. Prolonged infusion strategies were found to be a solution for rapidly cleared responsive probes in in vivo studues1-4 but other strategies for extending the tissue retention time of designed probes are still of need for desired applications. Here, we show the design of MRI probe, which form nano-assembly in live animal under physiological condition. A synthetic fluorinated-probe that can be used to detect labile Zn2+ in vitro by combining CEST and 19F-MRI (19F-iCEST)5-8 was detectable in vivo even 21 hours after its injection allowing longitudinal in vivo MRI studies. This design strategy can be adopted to any MRI application where longitudinal in vivo imaging is needed.
Method:
A fluorinated-chelate that is based on a dipicolylamine motif, which specifically binds Zn2+, was synthesized followed by its coupling to a BTA (benzene-1,3,5-tricarboxamide) backbone to obtain the tripod compound 1. 19F-NMR and 19F-iCEST (B1=2.3 µT/2 s) were performed on 9.4 T NMR at 25 oC and 37 oC. Nano-assembly formation was evaluated by dynamic light scattering (DLS),19F-NMR, diffusion NMR studies and by visualization at 25 oC and 37 oC. In vivo experiments were performed on 15.2 T MRI scanner after intracranial injection of 1 µL of compound 1 (75 mM in DMSO) to the CA3 region of the hippocampus in the mouse brain. In vivo 19F-NMR and 19F-MRI experiments were performed to study the sensor clearance rate from the live animal brain. For 19F-MRI experiment, 19F-RAREst sequence was used with the following parameters: TR/TE=4000/10.8 ms, RARE factor=16, FOV=22X22 mm2 (32x32 matrix).
Results:
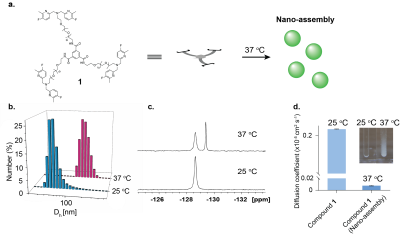
The nano-assembled Zn2+ responsive MRI probe compound 1 (Fig. 1a) with its 19F-dipicolylamine motifs as Zn2+ binding moieties was synthesized and characterized based on the hypothesis that tripod of this type tend to assemble to large architectures at elevated temperatures. Supramolecular structure formation of compound 1 at 37 oC in aqueous solution was confirmed by dynamic light scattering (DLS) measurements 19F-NMR and diffusion NMR. The DLS measurements showed the formation of 100 nm size assemblies only at 37 oC (Fig. 1b) and 19F-NMR showed an additional peak at elevated temperature, (upfield, Fig. 1c) that was assigned to the obtained large assemblies. The diffusion NMR studies confirmed that the additional upfield peak at the 19F-NMR is of the large assemblies showing x10 slower diffusion coefficient that is characteristic for large molecular entities (Fig. 1d). The formation of the large assemblies at 37 oC was observed by a naked-eye showing clear cloudiness of the heated solution (Fig. 1d, inset). The addition of Zn2+ to an aqueous solution of compound 1 resulted in an additional peak of Zn2+-1 complex in 19F-NMR spectrum (Δω=+5 ppm, Fig. 2a) and a characteristic 19F-iCEST spectrum showed a high CEST effect (Fig. 2a) from compound 1 in presence of labile Zn2+ at 37 oC revealing its potentiality to map the ion with MRI. Intracranially injection of compound 1 to a mouse brain was followed by in vivo 19F-MRS and 19F-MRI. The long retention time (>20 hours) of the injected probe was clearly evident by longitudinal in vivo 19F-MRS (Fig. 2b). In vivo 19F-MRI show the spatial distribution of compound 1 even 21 hours after its injection (Fig. 2c) confirming its prolonged retention time in the delivered region.
Conclusion:
We showed the design of MRI-responsive probe for Zn2+ sensing that forms nano-assemblies upon its injection to a live animal. We have demonstrated that the formed supramolecular structures is capable to detect Zn2+ when implementing the CEST principles in 19F-MRI (an approach termed 19F-iCEST). Moreover, we showed that upon its delivery, the 19F-MRI signal is preserved for more than 20 h after its injection. While the proposed approach was demonstrated here for a Zn2+ responsive probe that was designed for 19F-iCEST it can be generalized for the design of additional MRI responsive probes for applications that require long tissue-retention times for longitudinal in vivo studies.
Acknowledgements
No acknowledgement found.References
1. Savić, T.; Gambino, G.; Bokharaie, V. S.; Noori, H. R.; Logothetis, N. K.; Angelovski, G., Early detection and monitoring of cerebral ischemia using calcium-responsive MRI probes. Proceedings of the National Academy of Sciences 2019, 116 (41), 20666-20671.
2. Barandov, A.; Bartelle, B. B.; Williamson, C. G.; Loucks, E. S.; Lippard, S. J.; Jasanoff, A., Sensing intracellular calcium ions using a manganese-based MRI contrast agent. Nature Communications 2019, 10 (1), 897.
3. Gao, Y.; Shi, J.; Yuan, D.; Xu, B., Imaging enzyme-triggered self-assembly of small molecules inside live cells. Nature Communications 2012, 3 (1), 1033.
4. Lock, L. L.; Li, Y.; Mao, X.; Chen, H.; Staedtke, V.; Bai, R.; Ma, W.; Lin, R.; Li, Y.; Liu, G.; Cui, H., One-Component Supramolecular Filament Hydrogels as Theranostic Label-Free Magnetic Resonance Imaging Agents. ACS Nano 2017, 11 (1), 797-805.
5. Allouche-Arnon, H.; Tirukoti, N. D.; Bar-Shir, A., MRI-based Sensors for In Vivo Imaging of Metal Ions in Biology. Israel Journal of Chemistry 2017, 57 (9), 843-853.
6. Bar-Shir, A.; Gilad, A. A.; Chan, K. W. Y.; Liu, G.; van Zijl, P. C. M.; Bulte, J. W. M.; McMahon, M. T., Metal Ion Sensing Using Ion Chemical Exchange Saturation Transfer 19F Magnetic Resonance Imaging. Journal of the American Chemical Society 2013, 135 (33), 12164-12167.
7. Yuan, Y.; Wei, Z.; Chu, C.; Zhang, J.; Song, X.; Walczak, P.; Bulte, J. W. M., Development of Zinc-Specific iCEST MRI as an Imaging Biomarker for Prostate Cancer. Angewandte Chemie International Edition 2019, 58 (43), 15512-15517.
8. Tirukoti, N. D.; Avram, L.; Haris, T.; Lerner, B.; Diskin-Posner, Y.; Allouche-Arnon, H.; Bar-Shir, A., Fast Ion-Chelate Dissociation Rate for In Vivo MRI of Labile Zinc with Frequency-Specific Encodability. Journal of the American Chemical Society 2021, 143 (30), 11751-11758.
9. Hendrikse, S. I. S.; Su, L.; Hogervorst, T. P.; Lafleur, R. P. M.; Lou, X.; van der Marel, G. A.; Codee, J. D. C.; Meijer, E. W., Elucidating the Ordering in Self-Assembled Glycocalyx Mimicking Supramolecular Copolymers in Water. Journal of the American Chemical Society 2019, 141 (35), 13877-13886.
Figures