4418
CEST MRI Using Golden-Angle Cartesian Acquisition with Sparse Reconstruction1Biomedical Engineering and Imaging Institute and Department of Radiology, Icahn School of Medicine at Mount Sinai, New York, NY, United States
Synopsis
In this work, we proposed a novel dynamic CEST MRI framework, which employs golden-angle rotated variable-density Cartesian acquisition with multicoil compressed sensing reconstruction. It enables continuous data acquisition and retrospective reconstruction with desired temporal resolution. We have demonstrated the performance of this new framework for accelerated CEST MRI both in phantom and in human brain at 7T.
Introduction
Chemical exchange saturation transfer (CEST) imaging enables indirect detection of molecules that contain labile protons through their exchange with water. In addition to studying the CEST properties of many endogenous metabolites, injectable CEST contrast agents have been investigated recently, such as glucose and its derivatives.1-5 Injectable CEST agents offer a window into tracer dynamics. For example, the temporal change of dynamic glucose CEST (DGE) signal has been shown to reflect tissue perfusion and metabolism.3,6-8 However, to capture these dynamic changes, imaging speed and quality needs to be optimized. Very often, spatial resolution and coverage have to be reduced in order to achieve high temporal resolution. Besides, motion-related artifacts can also degrade image quality and obscure contrast changes.8-10 As a result, fast 3D CEST acquisition is highly desired for these studies. A few fast CEST methods have been proposed to acquire a 3D volume either with single-shot11,12 or multi-shot.13,14 In this study, we proposed a new 3D CEST MRI framework that combines golden-angle rotated variable-density Cartesian acquisition15 and multicoil compressed sensing reconstruction.Method
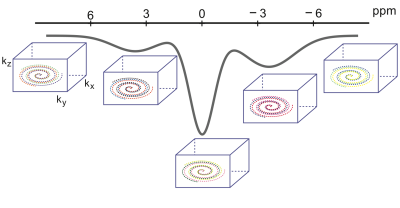
As illustrated in Figure.1, our approach employs variable density golden-angle spiral acquisition that is sampled directly on a Cartesian grid. Following the CEST preparation, one “shot”, which include a number of phase-encoding steps predefined by the user in the ky-kz plane, is acquired. Consecutive shots are rotated by the golden angle to allow for a uniform and continuous coverage of k-space. This feature enables dynamic imaging with retrospective reconstruction of arbitrary temporal resolution by grouping different numbers of consecutive shots into temporal frames, which would be particularly useful for dynamic CEST studies.The experiments were carried out at a Siemens Magnetom 7T scanner. Saturation was achieved by 20 gauss pulses, 50ms each and 0.5ms in between, B1rms powers of 0.7μT and 2.0μT. For image readout, 400 k-space lines were acquired per shot after the saturation, TE/TR/FA were set as 2.1ms/4.5ms/8°. In phantom study, 30 slices were acquired with thickness of 2mm within a field of view of 200mm2 and an in-plane resolution of 1.6mm2. 43 frequency offsets including 2 references at -167ppm and 41 offsets between -5 to 5ppm were acquired. For each frequency offset, 12 shots were acquired for retrospective down sampling evaluation. The inter-shot delay was set as 5s to allow sufficient relaxation recovery. The sampling trajectory was rotated by 137.5° between different shots, including those between each frequency offset. For human study, the sequence parameters are same as the phantom except for a larger slice thickness of 3mm. The B1rms power for saturation was 0.7μT. 23 frequency offsets (2 references, 21 between -5 to 5 ppm) were acquired, and 10 shots were acquired for each frequency offset. The inter-shot delay was set as 2s. The total scan time for brain imaging was 18min.
Multicoil compressed sensing16 was performed to reconstruct CEST images. Two reconstruction strategies were implemented. First, images were reconstructed using all the acquired shots with a sptatial constraint only. Second, images were reconstructed using only 3 shots for each measurement with a spatiotemporal constraint, which resulted in highly accelerated images.
Results and Discussion
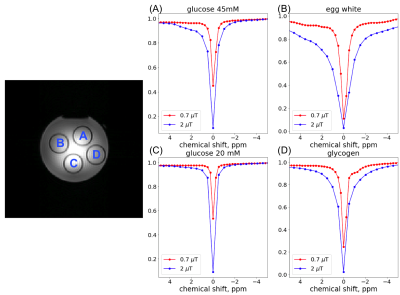
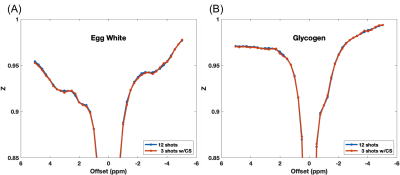
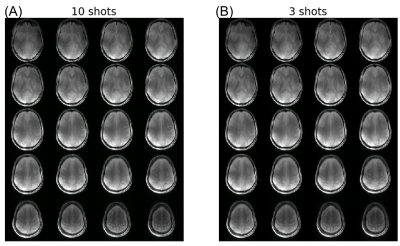
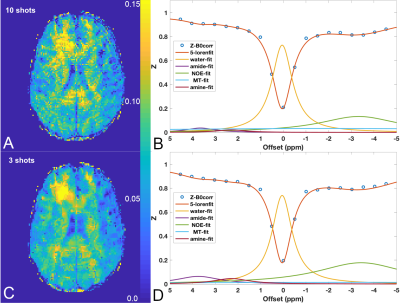
Figure.2 shows the Z-spectra from the 4 vials in the phantom at saturation power of 0.7μT and 2μT. Compared to the Z-spectra acquired at high power, the low power (0.7μT) Z-spectra showed more distinct resonances corresponding to the chemical exchange or Nuclear Overhauser Effect (NOE). These resonances are best observed in the egg white and the glycogen samples. We tested if reducing the number of shots used in image reconstruction can preserve these spectral features. Figure.3 shows the comparison of Z-spectra generated from images reconstructed with 12 and 3 shots. It can be seen that the spectra from images with 3 shots showed negligible differences compared to those from images with 10 shots. Figure.4 compares the brain images at -3.5ppm reconstructed using 10 shots and 3 shots. No notable image artifacts or visible differences were observed. For human brain study, we calculated NOE maps using the Lorentzian fitting method previous reported.11,17 Figure.5 shows the NOE maps from one slice and the corresponding Z-spectra from the same ROI in the frontal white matter. One can appreciate that by using 3 shots with multicoil compressed sensing reconstruction with a spatiotemporal constraint, the resulting NOE map is comparable to that generated using 10 shots using a spatial constraint only, and potentially less noisy. Both maps present some inhomogeneities in the posterior of the brain, possibly resulting from using customer-made work-in-progress dielectric pads. The acceleration can bring the acquisition time down to approximately 5.5min for 23 frequency offsets with full brain coverage, which allows CEST imaging to be integrated to clinical exams. Further work is needed to optimize the imaging acquisition and reconstruction method to improve the robustness against B0 and B1 inhomogeneities at ultra-high field and to test the method in dynamic CEST imaging studies, such as DGE MRI.Conclusion
We proposed a 3D CEST imaging using a golden-angle rotated variable-density Cartesian acquisition in combination with multicoil compressed sensing reconstruction. We have demonstrated that it is possible to obtain whole brain NOE maps within approximately 5.5min with this method, making it applicable to clinical applications.Acknowledgements
This work was supported by NIH R00 EB026312.References
(1) Chan, K. W. Y.; McMahon, M. T.; Kato, Y.; Liu, G.; Bulte, J. W. M.; Bhujwalla, Z. M.; Artemov, D.; van Zijl, P. C. M. Natural D-glucose as a biodegradable MRI contrast agent for detecting cancer. Magn Reson Med 2012, 68, 1764-1773.
(2) Walker-Samuel, S.; Ramasawmy, R.; Torrealdea, F.; Rega, M.; Rajkumar, V.; Johnson, S. P.; Richardson, S.; Goncalves, M.; Parkes, H. G.; Arstad, E.; Thomas, D. L.; Pedley, R. B.; Lythgoe, M. F.; Golay, X. In vivo imaging of glucose uptake and metabolism in tumors. Nat Med 2013, 19, 1067-1072.
(3) Xu, X.; Chan, K. W. Y.; Knutsson, L.; Artemov, D.; Xu, J.; Liu, G.; Kato, Y.; Lal, B.; Laterra, J.; McMahon, M. T.; van Zijl, P. C. M. Dynamic glucose enhanced (DGE) MRI for combined imaging of blood–brain barrier break down and increased blood volume in brain cancer. Magn Reson Med 2015, 74, 1556-1563.
(4) Jin, T.; Iordanova, B.; Hitchens, T. K.; Modo, M.; Wang, P.; Mehrens, H.; Kim, S.-G. Chemical exchange–sensitive spin-lock (CESL) MRI of glucose and analogs in brain tumors. Magn Reson Med 2018, 80, 488-495.
(5) Rivlin, M.; Tsarfaty, I.; Navon, G. Functional molecular imaging of tumors by chemical exchange saturation transfer MRI of 3-O-Methyl-D-glucose. Magn Reson Med 2014, 72, 1375-1380.
(6) Schuenke, P.; Paech, D.; Koehler, C.; Windschuh, J.; Bachert, P.; Ladd, M. E.; Schlemmer, H. P.; Radbruch, A.; Zaiss, M. Fast and Quantitative T1rho-weighted Dynamic Glucose Enhanced MRI. Sci Rep 2017, 7, 42093.
(7) Jin, T.; Mehrens, H.; Hendrich, K. S.; Kim, S. G. Mapping brain glucose uptake with chemical exchange-sensitive spin-lock magnetic resonance imaging. J Cereb Blood Flow Metab 2014, 34, 1402-1410.
(8) Herz, K.; Lindig, T.; Deshmane, A.; Schittenhelm, J.; Skardelly, M.; Bender, B.; Ernemann, U.; Scheffler, K.; Zaiss, M. T1ρ-based dynamic glucose-enhanced (DGEρ) MRI at 3 T: method development and early clinical experience in the human brain. Magnetic resonance in medicine : official journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine 2019, 82, 1832-1847.
(9) Zaiss, M.; Herz, K.; Deshmane, A.; Kim, M.; Golay, X.; Lindig, T.; Bender, B.; Ernemann, U.; Scheffler, K. Possible artifacts in dynamic CEST MRI due to motion and field alterations. J Magn Reson 2019, 298, 16-22.
(10) Xu, X.; Sehgal, A. A.; Yadav, N. N.; Laterra, J.; Blair, L.; Blakeley, J.; Seidemo, A.; Coughlin, J. M.; Pomper, M. G.; Knutsson, L.; van Zijl, P. C. M. d-glucose weighted chemical exchange saturation transfer (glucoCEST)-based dynamic glucose enhanced (DGE) MRI at 3T: early experience in healthy volunteers and brain tumor patients. Magn Reson Med 2020, 84, 247-262.
(11) Deshmane, A.; Zaiss, M.; Lindig, T.; Herz, K.; Schuppert, M.; Gandhi, C.; Bender, B.; Ernemann, U.; Scheffler, K. 3D gradient echo snapshot CEST MRI with low power saturation for human studies at 3T. Magn Reson Med 2019, 81, 2412-2423.
(12) Zaiss, M.; Ehses, P.; Scheffler, K. Snapshot‐CEST: Optimizing spiral‐centric‐reordered gradient echo acquisition for fast and robust 3D CEST MRI at 9.4 T. NMR Biomed 2018, 31, e3879-n/a.
(13) Khlebnikov, V.; Geades, N.; Klomp, D. W. J.; Hoogduin, H.; Gowland, P.; Mougin, O. Comparison of pulsed three-dimensional CEST acquisition schemes at 7 tesla: steady state versus pseudosteady state. Magnetic resonance in medicine : official journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine 2017, 77, 2280-2287.
(14) Krishnamoorthy, G.; Nanga, R. P. R.; Bagga, P.; Hariharan, H.; Reddy, R. High quality three-dimensional gagCEST imaging of in vivo human knee cartilage at 7 Tesla. Magnetic resonance in medicine : official journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine 2017, 77, 1866-1873.
(15) Cheng, J. Y.; Zhang, T.; Ruangwattanapaisarn, N.; Alley, M. T.; Uecker, M.; Pauly, J. M.; Lustig, M.; Vasanawala, S. S. Free-breathing pediatric MRI with nonrigid motion correction and acceleration. Journal of magnetic resonance imaging : JMRI 2015, 42, 407-420.
(16) Feng, L.; Srichai, M. B.; Lim, R. P.; Harrison, A.; King, W.; Adluru, G.; Dibella, E. V. R.; Sodickson, D. K.; Otazo, R.; Kim, D. Highly accelerated real-time cardiac cine MRI using k–t SPARSE-SENSE. Magn Reson Med 2013, 70, 64-74.
(17) Jones, C. K.; Huang, A.; Xu, J.; Edden, R. A. E.; Schär, M.; Hua, J.; Oskolkov, N.; Zacà, D.; Zhou, J.; McMahon, M. T.; Pillai, J. J.; van Zijl, P. C. M. Nuclear Overhauser enhancement (NOE) imaging in the human brain at 7 T. NeuroImage 2013, 77, 114-124.
Figures