4399
Joint cardiorespiratory 1D navigator triggering using left ventricular motion: improving T2w-FSE imaging for thoracic radiotherapy1Department of Radiotherapy, University Medical Center Utrecht, Utrecht, Netherlands
Synopsis
For MR-guided radiotherapy treatment planning of the thorax, cardiorespiratory triggered T2-weighted MRI images are desired. We investigate the usage of a navigator placed on the left ventricle to do cardiorespiratory triggering, and compare it to images obtained by placing the navigator on the liver-lung interface to do respiratory triggering. We scanned 5 healthy volunteers, making high-resolution scans with both navigators, and cine and 4D-MRI scans for reference. We found that cardiorespiratory triggering based on left ventricular motion is possible, and increases image quality, without increasing scan time and losing geometrical respiratory information.
Introduction
For MR-guided radiotherapy treatment planning in the thoracic region, T2-weighted (T2w) MRI images are used. The image quality of free-breathing images can be degraded due to cardiorespiratory motion-induced artifacts and/or blurring, which negatively affects structure delineations. To limit this motion, image acquisition can be done in a pre-defined window using triggering. T2w images require a long TR, making prospective triggering a useful method for motion management. Data acquisition will only start after trigger-acceptance, while the idle time assures a long TR. Navigators intertwined with the signal acquisition, requiring retrospective gating1, are deemed less efficient for T2w scans.Here, we propose to improve the T2w free-breathing scan quality in the thoracic region by placing a 1D image navigator on the left ventricle (LV), equivalent to the conventional liver-lung (LL) 1D navigator setup. We anticipate that LV motion holds information of both cardiac and respiratory motion based on earlier work2 and therefore makes it possible to perform cardiorespiratory triggering. We investigate the feasibility and the image quality improvement for T2w imaging of the thorax when using cardiorespiratory triggering based on motion of the LV versus motion of the LL-interface.
Methods
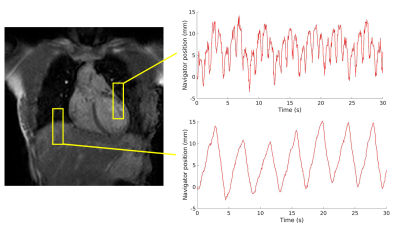
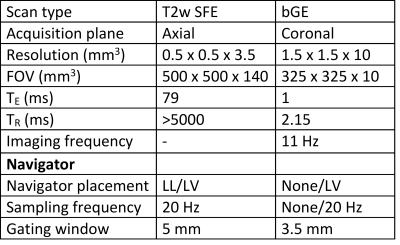
A 1D navigator is placed on the LV myocardial wall for prospective cardiorespiratory triggering of the image acquisition. Triggering is based on the position of the navigator signal. A single shot is acquired in the desired cardiorespiratory state (i.e., end-exhale and the heart in diastole). In Figure 1, the location of the navigator is visualized. Further scan parameters are summarized in Table 1.We scanned a total of 5 healthy volunteers on a 1.5T MR-simulator (Ingenia, Philips Healthcare, Best, NL). First, to quantify motion at the LL-interface and LV independently from the navigator, 2D cine images were obtained in 3 volunteers, covering the projected locations of the LV navigator. Additionally, the heart rhythm of 4 volunteers was recorded (ECG signal or peripheral pulse). The images were used to do a frequency decomposition of the LV motion, obtained by a template matching algorithm3, which was compared to a frequency decomposition of the cardiac signal and the LL-interface motion. Second, for 3 volunteers, cine images were obtained with and without LV gating, to quantify residual motion. For all volunteers, an LV-triggered T2w fast spin echo (FSE) PROPELLER scan (LV-PROPELLER) was acquired, as well as an LL-interface triggered T2w FSE PROPELLER scan (LL-PROPELLER). Lastly, a FSE 4D-MRI, which is sorted into 10 respiratory phases4, was acquired. To validate the geometry of the triggered T2w scans, rigid translation-only manual registrations between the PROPELLER scans and the end-exhale phase of the 4D-MRI were performed based on the location of the LL-interface. To quantify the image quality improvement of the LV-PROPELLER compared to the LL-PROPELLER, the sharpness of the LL-interface was determined, by finding the distance between the locations with 80% and 20% of maximum intensity in 5 separate line profiles in craniocaudal (CC) direction. The sharpness of the left ventricular wall was determined as well, by calculating the normalized image contrast gradient within the left ventricular myocardial wall. Voxels with a gradient >1 mm-1 are expected to be part of edges in the image.
Results
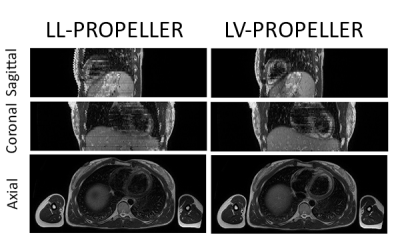
An example of the LL-PROPELLOR and LV-PROPELLOR images are shown for one volunteer in Figure 2.The average (min-max) scan time was 5:46 (4:45-8:02) min (LL-PROPELLER) and 5:51 (3:36-7:23) min (LV-PROPELLER).
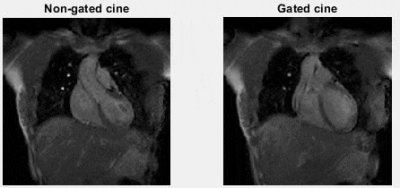
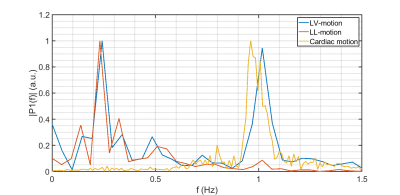
Based on the manual registrations, the average (min-max) CC distance between the LL-interface in the PROPELLER and the end-exhale phase of the 4D-MRI was 0.8 (0.0-2.0) mm (LL-PROPELLER) and 1.0 (0.0-2.0) mm (LV-PROPELLER) in caudal direction. The average (max-min) 80%-20% edge distance at the LL-interface was 6.24 (2.89-11.63) mm (LL-PROPELLER) and 6.35 (3.30-14.29) mm (LV-PROPELLER). The percentage of voxels at the left ventricular myocardial wall with a normalized image contrast gradient >1 mm-1 was 20% (LL-PROPELLER) and 25% (LV-PROPELLER). The average (min-max) root mean square error (RMSE) of the CC-motion of the left ventricle was 3.0 (1.6-4.3) mm in the non-gated cines, and 1.6 (0.9-2.5) mm in the gated cines. The average (min-max) RMSE of the CC-motion of the LL-interface was 3.8 (2.9-4.3) mm in the non-gated cines, and 1.9 (1.06-2.44) mm in the gated cines. The amount of residual motion is visualized in Figure 3. An example frequency spectrum is shown in Figure 4.
Discussion/Conclusion
Frequency analysis showed that the LV region holds information about both cardiac and respiratory motion. Gating scans based on the LV motion reduced LV as well as LL motion. Furthermore, we have shown that the LV triggering strategy increased the image sharpness compared to solely respiratory triggering using the LL-interface. Triggering on the LV or the LL-interface both results in images close to the desired end-exhale (note: we did not quantify distance from diastole in this abstract). Surprisingly, LV triggering did not result in longer scan times. We hypothesize that the high navigator sampling frequency is key here, as this enables scanning at each possible correct combination of end-exhale and diastole, though more investigation on this idea is warranted. We foresee, that the high-quality LV-triggered T2w images, which do not require the use of external triggering devices, can be used to aid radiotherapy planning and motion management strategies.Acknowledgements
MF Fast and K Keijnemans acknowledge funding by the Dutch Research Council (NWO) through project no. 17515 (BREATHE EASY).References
1. Rigie, D, Vahle, T, Zhao, B, Czekella, LJ., Frohwein, K, Boada, FE. Cardiorespiratory motion-tracking via self-refocused rosette navigators. Magn Reson Med. 2019; 81(5): 2947– 2958.
2. Nehrke K. and Manke D. Advanced Navigator Techniques, IJBEM 2000; 2(2):248-254.
3. Akdag O., Mandija S., Borman P., Alberts E., and Fast M. Feasibility of free breathing real-time cine-MRI for MR-guided Cardiac Radioablation on the Unity MR-linac, ISMRM 2021; abstract 4014.
4. Keijnemans K, Borman PTS, van Lier ALHMW, Verhoeff JJC, Raaymakers BW, Fast MF. Simultaneous multi-slice accelerated 4D-MRI for radiotherapy guidance. Phys Med Biol. 2021 Apr 28;66(9). 095014.
Figures