4393
Motion-corrected radial head MRI without any navigator, data redundancy or external tracking1Mallinckrodt Institute of Radiology, Washington University in St. Louis, SAINT LOUIS, MO, United States, 2Public Health Sciences, Washington University in St. Louis, SAINT LOUIS, MO, United States, 3Office of Research, Rice University, Houston, TX, United States, 4Division of Plastic and Reconstructive Surgery, Washington University in St. Louis, SAINT LOUIS, MO, United States, 5Mallinckrodt Institute of Radiology, Washington University in St. Louis, St. Louis, MO, United States
Synopsis
Patient motion is a common problem in MRI. Both prospective and retrospective schemes can be inefficient if they use navigators. Therefore, self-navigation without any data redundancy is preferable. In this study, we propose a simple and robust motion detection scheme that is free from stringent assumptions. This is especially important while imaging the head because the motion patterns are unpredictable. The corrected and uncorrected images were reviewed by two neuroradiologists in terms of osseous distinction (in reference to CT), sharpness and artifact-freeness. The method is able to increase osseous distinction and sharpness without increasing artifacts. This offers substantial clinical utility.
INTRODUCTION
For the magnetic resonance imaging (MRI) of the head, padding is of limited success in reducing motion. Sedation helps, but can result in adverse medical events, especially in children (1,2).Prospective motion correction methods make use of external tracking hardware (3) or navigators (4–6) to adjust imaging gradients in real time. External tracking equipment is expensive and requires maintenance. On the other hand, navigators reduce temporal efficiency, and can also alter the intensities and the contrast (6). Furthermore, prospective schemes are subject to the physical limitations of the gradient system.
Most retrospective methods utilize data redundancy (7,8) or acquire additional navigator data (9–11). Due to the reduced temporal efficiency of these approaches, self-navigated schemes utilizing non-Cartesian acquisitions are more favorable (12–14). Radial trajectories are more robust to motion than Cartesian trajectories (15), and also allow for the reconstruction of multiple temporal windows that can be used for tracking motion. The estimated motion parameters can then be applied directly in k-space (13,16–18) or in the image domain during an iterative reconstruction (19,20). Nonetheless, unlike respiratory motion, head motion is completely random. Therefore, a simple and robust method of motion detection prior to motion estimation is essential. Trajectory errors can degrade the performance of complicated schemes (13).
We previously developed a motion correction method called MUFFIN: MUlti-Frame Forward-modeled Image Numismatics (21), which uses a high-resolution hybrid radial acquisition and estimates rigid body transformations between fixed-duration temporal windows. However, since predetermined windows may not coincide with the actual motion states, we developed a novel, simplistic and robust motion detection scheme that derives the motion curve directly from the imaging data itself without any additional navigators. We evaluated the performance of the proposed method in unsedated pediatric patients.
METHODS
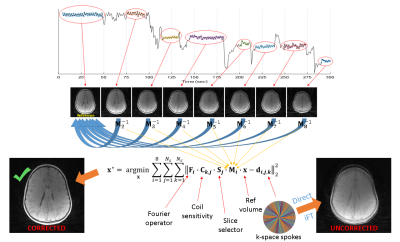
Upon Institutional Review Board approval, informed consent was obtained from the parent(s) of all participating children. MRI images were acquired using Prisma and Vida scanners (Siemens, Erlangen, Germany). A Fast Low-Angle Shot (FLASH) Golden-Angle 3D stack-of-stars radial VIBE sequence (22) was used that is Cartesian along the slice direction. The imaging protocol was as follows: TE/TR = 2.47 ms/4.84ms, Bandwidth = 410 Hz/pixel, 224 slices per slab, transverse orientation, Flip angle = 3-5°, Acquisition matrix = 320 × 320, Voxel size 0.6 x 0.6 x 0.8 mm and number of radial lines = 400 for a scan duration of about 5 minutes.Motion detection: The first 5 spokes were discarded to reach a steady state. After the inverse Fourier along the slice direction, the k-space center signal for each slice was obtained by a sum-of-squares (SoS) combination of all coil k-space centers. The slice showing the largest variation of SoS signal over time is the slice most sensitive to motion and hence its temporal SoS signal was used for guiding the motion correction process.
Determining motion frames: The SoS signal shows ripples due to gradient system imperfections. The rippling frequency is, however, simply half the golden section of the sampling frequency. Therefore, a stopband filter at this frequency was applied. After that, motion tracking was done by comparing each sample to the median level of its previous neighbors. If the deviation was more than 5% of the median level, it signaled a new motion state. Frames with less than 13 spokes (about 10 seconds) were discarded.
Motion estimation: The longest frame was chosen as the reference for image registration. In order to mitigate undersampling effects and considering the Nyquist sampling criterion for radial acquisitions, Hamming apodization was applied with an adaptive window length that was proportional to the number of spokes in each motion frame. These low-resolution volumes were then rigid-body registered onto the reference to derive the motion parameters.
Image reconstruction: Forward-modeled iterative optimization was used to obtain the corrected image. Figure 1 summarizes the full reconstruction scheme.
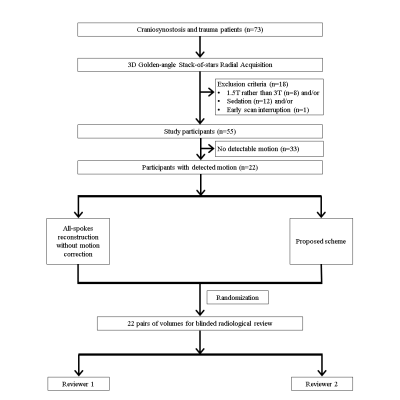
Image review: Out of 55 patients, 22 were motion-corrupted as detected by the algorithm. The corrected and uncorrected reconstructions of these cases were presented to two neuroradiologists (J.D. and M.S.G.) for an independent and blinded review on a 3-point scale with regard to osseous distinction relative to CT (for skull MRI purposes), sharpness and artifact-freeness. Figure 2 details the exclusion criteria and the review process.
Statistical analysis: Wilcoxon matched-pairs signed rank test was used per reviewer.
RESULTS
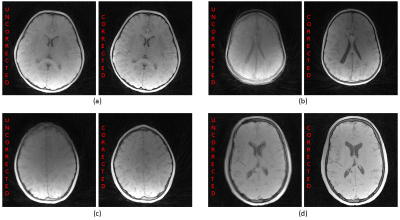
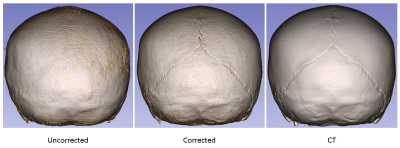
Figure 3 illustrates sample uncorrected and corrected reconstructions for four different patients. The recovery of fine details and sharpness is remarkable.Figure 4 demonstrates the 3D skull renderings of the patient in Figure 3 (a), in comparison with the gold-standard CT. The sutures were successfully recovered with motion correction.
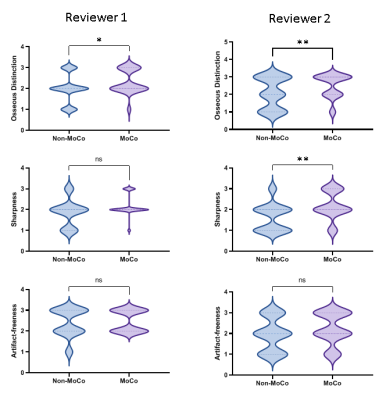
Figure 5 depicts the results of the Wilcoxon signed-rank test. The proposed scheme improves osseous distinction according to both reviewers and sharpness per one reviewer. Despite the possible discarding of spokes, the proposed scheme does not amplify artifacts.
DISCUSSION
The robustness of the proposed scheme is stemming from the simplicity of the motion detection approach. The limitation is that the patient needs to keep still during the acquisition of each spoke interval (0.75 sec).CONCLUSION
The proposed scheme can help substantially in the clinical imaging of pediatric and dementia populations. Furthermore, its success in skull imaging can save children from CT's ionizing radiation.Acknowledgements
No acknowledgement found.References
1. Coté CJ, Karl HW, Notterman DA, Weinberg JA, McCloskey C. Adverse sedation events in pediatrics: analysis of medications used for sedation. Pediatrics 2000;106:633–644.
2. Uffman JC, Tumin D, Raman V, Thung A, Adler B, Tobias JD. MRI Utilization and the Associated Use of Sedation and Anesthesia in a Pediatric ACO. J. Am. Coll. Radiol. JACR 2017;14:924–930.
3. Zaitsev M, Dold C, Sakas G, Hennig J, Speck O. Magnetic resonance imaging of freely moving objects: prospective real-time motion correction using an external optical motion tracking system. NeuroImage 2006;31:1038–1050 doi: 10.1016/j.neuroimage.2006.01.039.
4. van der Kouwe AJW, Benner T, Dale AM. Real-time rigid body motion correction and shimming using cloverleaf navigators. Magn. Reson. Med. 2006;56:1019–1032.
5. White N, Roddey C, Shankaranarayanan A, et al. PROMO – Real-time Prospective Motion Correction in MRI using Image-based Tracking. Magn. Reson. Med. Off. J. Soc. Magn. Reson. Med. Soc. Magn. Reson. Med. 2010;63:91–105 doi: 10.1002/mrm.22176.
6. Tisdall MD, Hess AT, Reuter M, Meintjes EM, Fischl B, van der Kouwe AJW. Volumetric navigators for prospective motion correction and selective reacquisition in neuroanatomical MRI. Magn. Reson. Med. 2012;68:389–399.
7. Pipe JG. Motion correction with PROPELLER MRI: Application to head motion and free-breathing cardiac imaging. Magn. Reson. Med. 1999;42:963–969.
8. Liu C, Bammer R, Kim D, Moseley ME. Self-navigated interleaved spiral (SNAILS): Application to high-resolution diffusion tensor imaging. Magn. Reson. Med. 2004;52:1388–1396 doi: 10.1002/mrm.20288.
9. Ehman RL, Felmlee JP. Adaptive technique for high-definition MR imaging of moving structures. Radiology 1989;173:255–263 doi: 10.1148/radiology.173.1.2781017.
10. Johnson PM, Liu J, Wade T, Tavallaei MA, Drangova M. Retrospective 3D motion correction using spherical navigator echoes. Magn. Reson. Imaging 2016;34:1274–1282 doi: 10.1016/j.mri.2016.06.006.
11. Kecskemeti S, Samsonov A, Velikina J, et al. Robust Motion Correction Strategy for Structural MRI in Unsedated Children Demonstrated with Three-dimensional Radial MPnRAGE. Radiology 2018;289:509–516 doi: 10.1148/radiol.2018180180.
12. Welch EB, Rossman PJ, Felmlee JP, Manduca A. Self-navigated motion correction using moments of spatial projections in radial MRI. Magn. Reson. Med. 2004;52:337–345 doi: 10.1002/mrm.20151.
13. Anderson III AG, Velikina J, Block W, Wieben O, Samsonov A. Adaptive retrospective correction of motion artifacts in cranial MRI with multicoil three-dimensional radial acquisitions. Magn. Reson. Med. 2013;69:1094–1103 doi: 10.1002/mrm.24348.
14. Feng L, Axel L, Chandarana H, Block KT, Sodickson DK, Otazo R. XD-GRASP: Golden-angle radial MRI with reconstruction of extra motion-state dimensions using compressed sensing. Magn. Reson. Med. 2016;75:775–788.
15. Glover GH, Pauly JM. Projection Reconstruction Techniques for Reduction of Motion Effects in MRI. Magn. Reson. Med. 1992;28:275–289.
16. Loktyushin A, Nickisch H, Pohmann R, Schölkopf B. Blind retrospective motion correction of MR images. Magn. Reson. Med. 2013;70:1608–1618.
17. Cordero-Grande L, Teixeira RPAG, Hughes EJ, Hutter J, Price AN, Hajnal JV. Sensitivity Encoding for Aligned Multishot Magnetic Resonance Reconstruction. IEEE Trans. Comput. Imaging 2016;2:266–280.
18. Graedel NN, McNab JA, Chiew M, Miller KL. Motion correction for functional MRI with three‐dimensional hybrid radial‐Cartesian EPI. Magn. Reson. Med. 2017;78:527–540.
19. Cruz G, Atkinson D, Buerger C, Schaeffter T, Prieto C. Accelerated motion corrected three-dimensional abdominal MRI using total variation regularized SENSE reconstruction. Magn. Reson. Med. 2016;75:1484–1498.
20. Cruz G, Atkinson D, Henningsson M, Botnar RM, Prieto C. Highly efficient nonrigid motion-corrected 3D whole-heart coronary vessel wall imaging. Magn. Reson. Med. 2017;77:1894–1908.
21. Eldeniz, Cihat, Jammalamadaka, Udayabhanu, Skolnick, Gary B., Commean, Paul K., Patel, Kamlesh B., An, Hongyu. Skull MRI with MUFFIN: MUlti-Frame Forward-modeled Image Numismatics. In Proceedings of the 29th Annual Meeting of ISMRM, Virtual, 2021. Abstract #522.
22. Peters DC, Korosec FR, Grist TM, et al. Undersampled projection reconstruction applied to MR angiography. Magn. Reson. Med. 2000;43:91–101.
Figures