4385
Saturated Multi-delay Arterial Spin Labeling (SAMURAI) Technique for Simultaneous Perfusion and T1 Quantification of Kidneys1Center for Biomedical Imaging Research, Department of Biomedical Engineering, School of Medicine Tsinghua University, Beijing, China, 2Institute of Science and Technology for Brain-Inspired Intelligence, Fudan University, Shanghai, China, 3Philips Healthcare, Shanghai, China
Synopsis
We proposed a Saturated Multi-delay Arterial Spin Labeling (SAMURAI) technique with a correspondingly modified kinetic model to achieve simultaneous acquisitions of RBF, aBAT, tBAT and T1 map in kidney with a single scan. SAMURAI provided T1 map with excellent correlation (R2=0.976) compared with IR-SE. Compared with multi-TI FAIR, SAMURAI provided equally reliable ASL and T1 quantification results (ICC: 0.875-0.958) with excellent scan-rescan repeatability (ICC: 0.905-0.992) and significantly reduced scan time (45’ vs 4’6’’ for 9 TIs).
Introduction
Arterial spin labeling (ASL) and T1 mapping are promising tools for diagnosis, prognosis prediction, and treatment monitoring in many kidney diseases in terms of function and structure. The corresponding quantitative parameters including renal blood flow (RBF), arterial bolus arrival time (aBAT), tissue BAT (tBAT) and T1-map enable early detection of kidney diseases1,2. However, acquiring above multiparametric information usually needs repeated scans for multi-TI ASL and extra T1 mapping sequence, which prolongs scan time and limits clinical practicality. In this study, we proposed a Saturated Multi-delay Arterial Spin Labeling (SAMURAI) technique with a correspondingly modified kinetic model to achieve simultaneous acquisitions of RBF, aBAT, tBAT and T1 map in kidney with a single scan.Methods
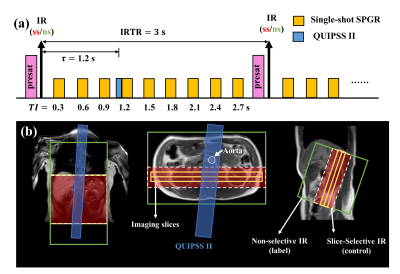
Sequence design: The SAMURAI sequence consists of a pre-saturation pulses at imaging slice followed by a nonselective (ns) or slice-selective (ss) inversion pulse for ASL acquisition and Look-Locker SPGR readout (Fig 1). Nine TIs of the Look-Locker SPGR sampling series ranged from 0.3 to2.7s with a step size of 0.3s. QUIPSS II pulses3 were performed at bolus duration $$$\tau=1.2s$$$. Other imaging parameters include: TE/TR 1.96/3.9ms, TFE factor 30, flip angle 6°, slice thickness 6mm, 40 averages, and CS-SENSE factor 3.2.Phantom Experiment: Phantom experiment was performed on a 3T MR scanner (Ingenia, Philips Healthcare, Best, The Netherlands) with a 32-channel head coil. The SAMURAI (FOV 135×71mm2, in-plane resolution 1.6×1.6mm2, slice thickness 6mm) and a series of standard IR-SE sequences (14 TIs: 0.1:0.1:1/1.5/2/2.5/3s, TE/TR 9.3/10000ms, same FOV and resolution) were performed for T1-mapping validation.
In-vivo Experiment: Eleven healthy volunteers (5 males, mean age: 25.0±2.1years) and one patient (male, 23-year-old) with renal calculus were recruited for MRI experiments with informed consent. The experiments were conducted on the same MR scanner with a 16-channel torso coil and a 12-channel posterior coil. The SAMURAI sequence was acquired twice (FOV 288×288mm2, in-plane resolution 3×3mm2, slice thickness 6mm, scan time 4’6’’) under untrained free breathing. A series of multi-TI FAIR sequences4 with the same parameters as SAMURAI except for utilization of the Look-Locker strategy (45’ for 9 TIs) were performed for validation of T1 and ASL quantification.
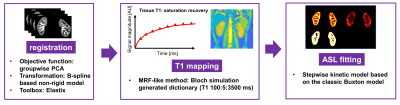
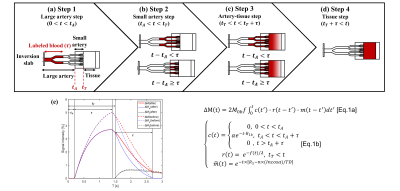
Image Analysis: All analyses were performed on MATLAB (Mathworks, Natick) as shown in Fig 2. To address the respiratory motion, all data sets were registered by a retrospective motion correction on Elastix5 with a non-rigid principle component analysis-based (PCA-based) groupwise registration6. T1-map of SAMURAI was obtained by dictionary-based methods, with the dictionary generated by Bloch simulation (T1 100:5:3500ms) and minimum Euclidean distance for the best matching. For ASL quantification including RBF, aBAT, and tBAT, a stepwise kinetic model was proposed based on the classic Buxton model7, taking into account the process that blood firstly flows into arteries at the imaging slab and then into the kidney tissue. Besides, impact on ASL signal due to Look-Locker sampling scheme were also corrected in the proposed model (Fig 3).
Results
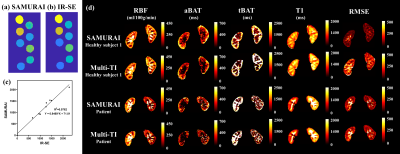
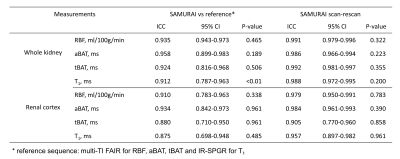
The proposed SAMURAI technique can provide accurate T1 map (Fig 4a) with excellent correlation (R2=0.976) compared with IR-SE (Fig 2b). Compared with multi-TI FAIR, the SAMURAI technique provided equally reliable ASL and T1 quantification results (ICC: 0.875-0.958) with excellent scan-rescan repeatability (ICC: 0.905-0.992) (Fig 5) and significantly reduced scan time (45’ vs 4’6’’ for 9 TIs).Discussion and Conclusion
This study proposed the SAMURAI technique for simultaneous acquisition of RBF, aBAT, tBAT and T1 map in kidney with reliable ASL and T1 quantification, excellent repeatability and significantly reduced scan time compared to traditional techniques.Acknowledgements
None.References
1. Wolf M, de Boer A, Sharma K, et al. Magnetic resonance imaging T1- and T2-mapping to assess renal structure and function: a systematic review and statement paper. Nephrol Dial Transplant. 2018;33(suppl_2):ii41-ii50.
2. Zhang JL, Lee VS. Renal perfusion imaging by MRI. J Magn Reson Imaging. 2020;52(2):369-79.
3. Wong EC, Buxton RB, Frank LR. Quantitative imaging of perfusion using a single subtraction (QUIPSS and QUIPSS II). Magn Reson Med. 1998;39(5):702-8.
4. Kim SG. Quantification of relative cerebral blood flow change by flow-sensitive alternating inversion recovery (FAIR) technique: application to functional mapping. Magn Reson Med. 1995;34(3):293-301.
5. Klein S, Staring M, Murphy K, Viergever MA, Pluim JP. elastix: a toolbox for intensity-based medical image registration. IEEE Trans Med Imaging. 2010;29(1):196-205.
6. Huizinga W, Poot DH, Guyader JM, et al. PCA-based groupwise image registration for quantitative MRI. Med Image Anal. 2016;29:65-78.
7. Buxton RB, Frank LR, Wong EC, Siewert B, Warach S, Edelman RR. A general kinetic model for quantitative perfusion imaging with arterial spin labeling. Magn Reson Med. 1998;40(3):383-96.
Figures