4377
Blockade of lymphatic drainage to deep cervical lymph nodes impacts CSF flow and brain morphometry1Anesthesiology, Yale University, New Haven, CT, United States, 2Department of Applied Mathematics and Statistics, Stony Brook University, Stony Brook, NY, United States, 3Department of Radiation Oncology, Weill Cornell Medicine, New York, NY, United States, 4Department of Pathology and Immunology, Washington University, St. Louis, MO, United States
Synopsis
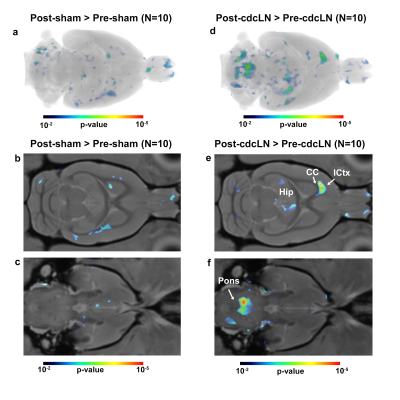
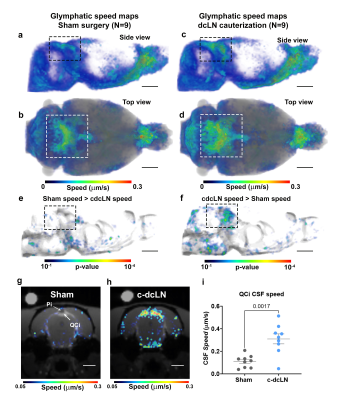
The altered CNS fluid flow dynamics and homeostasis mechanisms in response to blockade of lymphatic drainage to deep cervical lymph nodes (dcLN) is poorly understood. We used multi-modality MRI and computational fluid dynamics approach to study the same in rats with electrocauterized afferent lymphatic vessels of dcLN. The brain morphometry of Cauterized-dcLN rats showed localized volume expansion in Pons, Hippocampus and Corpus-callosum, while glymphatic speed maps showed hyperdynamic CSF flow in ambient and quadrigeminal cisterns. These results clearly show that the blockade of CSF drainage to dcLN alters CNS fluid homeostasis/flow dynamics, long-term may result in waste accumulation and neurodegeneration.
INTRODUCTION
There has been a growing interest in the functional drivers of fluid homeostasis in the central nervous system (CNS)1. The scientific interest has evolved with recent studies showing that circulation of cerebrospinal fluid (CSF) through the glymphatic system and meningeal lymphatics facilitates waste disposal and immune surveillance of the CNS 2,3. Indeed, aging, besides being linked to cognitive dysfunction, is also associated with a decrease in glymphatic-lymphatic system function 4,5. The meningeal lymphatic vessels drain waste solutes from the CNS via the glymphatic-lymphatic system primarily to the deep cervical lymph nodes (dcLN)6. Notably, ablation of meningeal lymphatic vessels has been shown to increase the Ab burden in the brain of mice models of Alzheimer’s disease4. Although studies have shown that blockade of lymphatic drainage to dcLN in rodents does not affect intra-cranial pressure (ICP)4, the homeostatic mechanisms in place to prevent fluid accumulation and tissue damage remain poorly understood. Here we used multi-modality MRI and computational fluid dynamics (CFD) analysis to report on changes in CSF flow and glymphatic transport along with brain morphometry from rats exposed to blockade of drainage to dcLN in comparison to sham controls.METHODS
All animal experiments were approved by the Yale University IACUC. MRI imaging acquisitions were performed on a Bruker 9.4T/16 MRI equipped with a volume transmit/receive or surface receive only coils interfaced with Paravision 6. Three-month-old female SD rats either underwent: 1) sham surgery (N=20) which entailed exposing the dcLN and gently stroking each node with a sterile gauze swab or 2) electrocauterization of the afferent lymphatics to the dcLN (a.k.a. ‘c-dcLN’) bilaterally (N=20) via a small neck incision. All rats received analgesia with meloxicam and buprenorphine pre-operatively and 48 hrs post-surgery. The following groups were studied: Cohort 1: Sham (N=10) and c-dcLN (N=10) rats each underwent two MRI brain morphometry scans 7-days apart pre- and post-surgery; Cohort 2: Sham (N=10) and c-dcLN (N=10) underwent DCE-MRI for CSF flow dynamics and glymphatic transport measurements 4-6 days post-surgery. All rats were scanned while anesthetized with dexmedetomidine supplemented with isoflurane7. For deformation-based morphometry (DBM), a 3D proton density-weighted images were acquired using SPGR sequence (TR/TE/FA/Ave = 15ms/4ms/7°/4, spatial resolution = 0.23x0.23x0.23 mm). The image registration and voxel-wise statistical analysis were performed using SPM12. For DCE-MRI a small catheter was first implanted in CSF via the cisterna magna and gadoteric acid (Gd-DOTA) was infused to produce dynamic measurement of glymphatic transport via CFD analysis based on regularized optimal mass transport (rOMT) theory as described in Koundal et al.,7.RESULTS & DISCUSSION
Seeking to identify possible brain areas of fluid dyshomeostasis caused by blocking drainage to the dcLN, we first investigated volumes changes in the brain following sham or c-dcLN surgery. The DBM analysis for c-dcLN > baseline revealed multiple areas in the pons, hippocampus, and corpus callosum with significant volume ‘expansion’ implying the presence of focal tissue edema (Figs. 1a-c). In contrast, for the sham cohort, only subtle changes for the corresponding analysis were noted (Figs. 1d-f). Prompted by previous reports on glymphatic transport decreases following ablation of the dorsal meningeal lymphatic network in mice4, we investigated CSF flow and glymphatic system function in rats after blocking drainage to dcLN. Figs. 2 shows population-averaged CSF and glymphatic speed maps from the sham (Figs. 2a, b) and c-dcLN (Figs. 2c, d) rats. Note that the speed maps at the voxel level capture two distinct features: (1) CSF and glymphatic volume ‘flux’ of solute transport over ~2h, and (2) speed of CSF and glymphatic transport. The blue and red colors represent slow and fast solute speed, respectively. The voxel based SPM analysis of the speed maps revealed significantly greater speed in CSF rich areas associated with the ambient and quadrigeminal cisterns (QCi) for the c-dcLN > sham condition (Figs. 2e, f). The post-hoc statistical analysis of the QCi area showed that the CSF speed was increased by ~60% in the c-dcLN compared to sham rats (Figs. 2g-i).CONCLUSIONS
We showed that arrest of drainage to dcLN caused perturbation of CNS fluid homeostasis in distinct areas of white matter rich regions but not globally, which further validates reports that ICP is not affected in this condition. However, our CFD analysis revealed that CSF flow speed increased significantly at the level of the QCi, which is positioned directly underneath the confluence of sinuses. This observation is particularly intriguing as the confluence of sinuses serves as an exit hot-spot for waste solute drainage via meningeal lymphatics to the dcLN3 and implies the presence of ‘stasis’ which long term may lead to waste accumulation and neurodegeneration.Acknowledgements
The authors thank Peter Brown of MRRC (Magnetic Resonance Research Center) at Yale University for coil development and support. Funding: This work was supported by a grant from the National Institutes of Health/ NCCIH R01AT011419References
1-Benveniste, H. et al. The glymphatic system and its role in cerebral homeostasis. J Appl Physiol (1985) 129, 1330-1340, doi:10.1152/ japplphysiol.00852.2019 (2020).
2-Kanamori, M. & Kipnis, J. Meningeal lymphatics "drain" brain tumors. Cell Res 30, 191-192, doi:10.1038/s41422-020-0286-9 (2020).
3-Rustenhoven, J. et al. Functional characterization of the dural sinuses as a neuroimmune interface. Cell 184, 1000-1016 e1027, doi:10.1016/j.cell.2020.12.040 (2021).
4-Da Mesquita, S. et al. Functional aspects of meningeal lymphatics in ageing and Alzheimer's disease. Nature 560, 185-191, doi:10.1038/s41586-018-0368-8 (2018).
5-Kress, B. T. et al. Impairment of paravascular clearance pathways in the aging brain. Ann Neurol 76, 845-861, doi:10.1002/ana.24271 (2014).
6-Louveau, A. et al. Structural and functional features of central nervous system lymphatic vessels. Nature 523, 337-341, doi:10.1038/nature14432 (2015).
7-Koundal, S. et al. Optimal Mass Transport with Lagrangian Workflow Reveals Advective and Diffusion Driven Solute Transport in the Glymphatic System. Sci Rep 10, 1990, doi:10.1038/s41598-020-59045-9 (2020).
Figures