4373
Preliminary Study of the Uncertainty for RF-induced Heating Assessment of Subcutaneous and Partially Implanted Devices during MRI Exposure1Lanzhou University, Lanzhou, China, 2Shanghai NeuraZing Co., Ltd, Shanghai, China
Synopsis
This work intends to provide a preliminary evaluation of the uncertainty produced by current Tier 3 approach during the assessment of subcutaneous and partially implanted devices. The transfer function of a 600 mm long DBS and guidance wire for Interventional MRI are derived under homogeneous and heterogeneous tissue environments, respectively. The resulting power deposition using different transfer function are evaluated and compared. The results show that using current Tier 3 approach may lead to potential large underestimation of the power deposition for subcutaneous and partially implanted devices, methods which can overcome or reduce these uncertainty are needed.
Introduction
There are many practical limitations and restrictions associated with the assessment the radio-frequency (RF) induced heating of active implantable medical devices (AIMDs) in patients undergoing magnetic resonance imaging (MRI) examination according to the four-tier approach proposed in Clause 8 of ISO/TS1. Until now, Tier 3 has been the most commonly used approach to perform a comprehensive RF-induced heating assessment1,2. As it can significantly reduce the numerical complexity of multi-scale full-wave evaluation in Tier 4 by separating the implant-RF interaction (characterized with an implant transfer function) from the human-RF interaction (characterized by a tangential electric field (Etan) induced along implant trajectories in vivo). The accuracy of the Tier 3 assessment is heavily dependent on both the accuracy of implant RF transfer function and Etan. Due to practical limitations in the derivation of the implant transfer function based on current methods3,4, the implant transfer function in heterogeneous tissue models cannot be realistically obtained through numerical modeling. Therefore, in current Tier 3 assessments, the AIMD transfer functions are usually derived by means of an in vitro experimental method4 in homogeneous medium with dielectric properties defined in ISO/TS 109741 to mimic the inhomogeneous in vivo tissue environment. This approach works fine with fully-implanted devices, such as pacemaker, where the implants are fully surrounded by the human tissue environment. However, for subcutaneous implanted device, such as deep brain stimulator (DBS), or partially implanted device, such as guide wires for interventional MRI, this approach may produce large estimation uncertainties. To provide a preliminary evaluation of the uncertainty caused by the current Tier 3 approach during the evaluation of the subcutaneous or partially implanted devices, the transfer function of a 600 mm DBS and guide wire are compared under homogeneous and heterogeneous medium in this work.Method
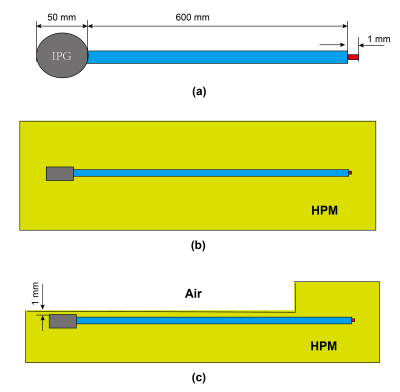
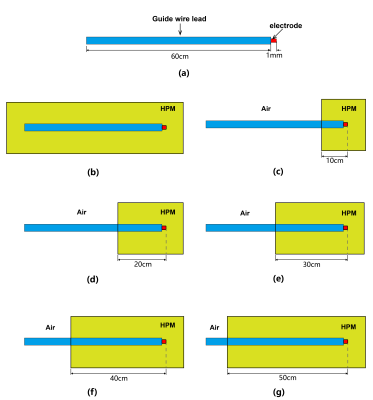
A 600-mm long implant lead with a 50-mm diameter IPG is used as a generic DBS, the wire diameter is 0.8 mm, insulation thickness is 0.6 mm, the length of the electrode is 1 mm as shown in Figure 1(a). To derive the transfer function of the generic DBS, the device is submerged in two representative scenarios: (1)homogeneous high permittivity medium (HPM) with σ = 0.47 S/m, relative permittivity εr of 78 as shown in Figure 1(b). (2) heterogeneous medium as shown in Figure 1(c), where the 100 mm lead segment starting from the electrode is fully immersed in the homogeneous HPM, while the remaining parts are placed near the HPM-Air boundary, with 1 mm distance from the IPG top to the HPM-Air boundary. The heterogeneous scenario is designed to mimic a typical DBS clinical position where the electrode is deep inside the brain, while the leads are in the subcutaneous region.Next, a 600 mm long implant lead with wire diameter of 0.8 mm, and insulation thickness of 0.6 mm is designed as a generic interventional MRI guide wire as shown in Figure 2(a). The transfer function of the guide wire is derived under both homogeneous and heterogeneous conditions as shown in Figure 2. In the homogeneous condition (Figure 2(b)), the guide wire is fully immersed in HPM with σ = 0.47 S/m, relative permittivity εr of 78. For the heterogeneous condition, 5 different scenarios are designed as shown in Figure 2(c)-(g), where the guide wire is partially implanted in the HPM with segment length of 10cm, 20cm, 30cm, 40cm and 50 cm, respectively. These heterogeneous medium conditions are referred as 10 cm HPM, 20 cm HPM, 30 cm HPM, 40 cm HPM, and 50 cm HPM, respectively. The transfer functions are derived numerically using computational platform Sim4Life V6.2.
Results
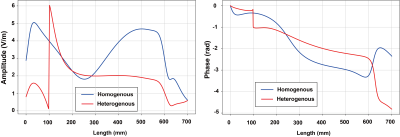
Figure 3 shows the normalized transfer function of DBS under homogeneous and heterogeneous condition with transfer function satisfying the following normalization condition:$$ (\int h(l)dl)(\int h(l)dl)^* = 1$$
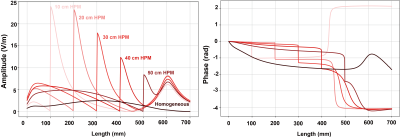
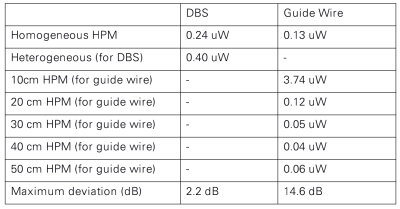
where h(l) is the normalized transfer function. Figure 4 shows the normalized transfer function of the generic guide wire under homogeneous and heterogeneous conditions, same normalization as DBS is applied here. The calibrated scaling factor of the transfer function for DBS and guide wire are summarized in Figure 5, respectively. It can be also treated as the power deposition of the generic DBS under a homogeneous incident condition with tangential electrical field along the implant (Etan) equals 1 V/m using the following equation:
$$P_{dep} = W_{0}(\int h(l)E_{tan}(l)dl)(\int h(l)E_{tan}(l)dl)^*$$
Where W0 is the scaling factor of the transfer function, and Etan = 1 V/m is the tangential electric field along the implant routing. From this point of view, the uncertainty caused by the medium condition is about 2.2 dB for the generic DBS, and 14.6 dB for guide wire.
Conclusion
This work provides a preliminary evaluation of the uncertainty produced by current Tier 3 approach during the RF heating evaluation of subcutaneous and partially implanted medical devices. The results show that the traditional Tier 3 approach using homogeneous medium to represent the special tissue environment of the subcutaneous and partially implanted medical devices can lead to large underestimation of the RF-induced power deposition. Further evaluation under more realistic in vivo exposure conditions is needed to provide a more comprehensive uncertainty quantification.Acknowledgements
This work is supported by National Natural Science Foundation of China (Grant No. 6210010061). The authors would like to thank Zurich Medtech (ZMT, Zurich, Switzerland) for providing Sim4Life for scientific use.References
1. ISO/TS. 10974:2012, Requirements for the safety of magnetic resonance imaging for patients with an active implantable medical device. DRAFT 2016.
2. Cabot E, Lloyd T, Christ A et al. Evaluation of the RF heating of a generic deep brain stimulator exposed in 1.5 T magnetic resonance scanner. Bioelectromagnetics 2013;34(2):-0197-8462.
3.Park S.M, Kamondetdacha R, and Nyenhuis J.A. Calculation of MRI-induced heating of an implanted medical lead wire with an electric field transfer function. J Magn Reson Imaging 2007;26(5):1278-1285.
4. Zastrow E, Capstick M and Niels K. Experimental systems for RF-heating characterization of medical implants during MRI. ISMRM 2016.
Figures