4371
A Shielding-free Ultra-low-field 0.055T Brain MRI Scanner for Accessible Healthcare1Laboratory of Biomedical Imaging and Signal Processing, The University of Hong Kong, Hong Kong SAR, China, 2Department of Electrical and Electronic Engineering, The University of Hong Kong, Hong Kong SAR, China, 3School of Biomedical Sciences, Li Ka Shing Faculty of Medicine, The University of Hong Kong, Hong Kong SAR, China, 4Department of Diagnostic Radiology, Li Ka Shing Faculty of Medicine, The University of Hong Kong, Hong Kong SAR, China, 5Department of Surgery, Li Ka Shing Faculty of Medicine, The University of Hong Kong, Hong Kong SAR, China, 6Department of Medicine, Li Ka Shing Faculty of Medicine, The University of Hong Kong, Hong Kong SAR, China
Synopsis
In this study, we describe an ultra-low-field brain MRI scanner that operates using a standard AC power outlet and is low cost to build. Using a permanent 0.055 Tesla Samarium-cobalt magnet and deep learning for cancellation of electromagnetic interference, it requires neither magnetic nor radiofrequency shielding cages. We also successfully implement four standard clinical neuroimaging protocols (T1W, T2W, FLAIR like, and DWI) and demonstrate human brain imaging on this system. This proof-of-concept work will advance the development of a new class of MRI technologies to democratize MRI for low and middle income countries and increase MRI accessibility in healthcare.
Introduction
MRI is intrinsically superior to other imaging modalities because it is non-invasive, non-ionizing, inherently quantitative and multi-parametric. However, MRI accessibility is low and extremely inhomogeneous worldwide1,2. Recently, there has been an impetus to develop low-cost MRI technologies at ultra-low-field (ULF) strengths2-9 for potential point-of-care applications. These studies indicate the exciting possibility of generating brain images with low-cost hardware, though the imaging versatility and image quality remain a challenge.In this study, we describe an ULF brain MRI scanner that operates using a standard AC power outlet and is low cost to build. Using a permanent 0.055T magnet and deep learning cancellation of electromagnetic interference (EMI), it requires neither magnetic nor RF shielding cages. The scanner is compact, potentially mobile, and acoustically quiet during scanning. We implement and demonstrate four most critical clinical neuroimaging protocols (T1W, T2W, FLAIR like, and DWI) on this system.
Methods
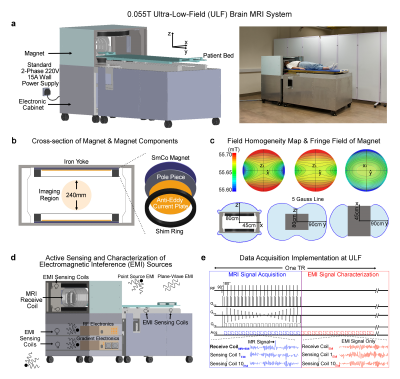
Magnet design: System was based on a compact two-pole 0.055T SmCo magnet with low temperature coefficient (Sm2Co17-22LTC). Other key magnet components included an iron yoke (iron 99.9% pure), polar pieces (iron 99.9% pure), anti-eddy current plates made from silicon steel (DW310-35), and passive shimming rings made from low carbon steel (Q235) (Figure 1A, B). The final 0.055T field, corresponding to 2.32MHz proton frequency, had an inhomogeneity <250ppm peak-to-peak over 240mm DSV. 5 Gauss fringe field was very small (Figure 1C).Gradient/RF subsystems: Gx and Gy gradient coils were unshielded, whereas Gz coil was actively shielded. The resistances of Gx, Gy, and Gz coils were 47.0mΩ, 45.5mΩ and 75.5mΩ, and their inductances were 120.1µH, 98.9µH and 88.2µH, respectively. The sensitivities of Gx, Gy, and Gz coils were 17.3mT/m/100A, 17.5mT/m/100A and 12.2mT/m/100A, respectively. The non-linearity of the gradient field was within 5% over 240mm DSV with maximum gradient of 15mT/m. Gradient coils were driven by a PCI GA150 gradient amplifier. Separate 2-plate RF transmit coil and 1-channel solenoid receive coils were employed10,11.
Deep learning EMI cancellation for shielding-free MRI (Figure 1D): Ten small resonant EMI sensing coils were fabricated and placed in the vicinity of the patient head holder, patient bed, and inside the electronic cabinet. These were used to detect pure EMI signals from both external environment and those generated internally by console/gradient/RF electronics during MRI scanning. Signals detected by these EMI sensing coils and main MRI receive coil within EMI signal characterization window (Figure 1E) retrospectively were used to train a CNN model. This model could then predict the EMI signal component in MRI receive coil signal for each frequency encoding line within MRI signal acquisition window based on the EMI signals simultaneously detected by EMI sensing coils. This predicted EMI signal component was subsequently subtracted or removed from the MRI receive coil signals, creating EMI-free k-space data prior to image reconstruction.
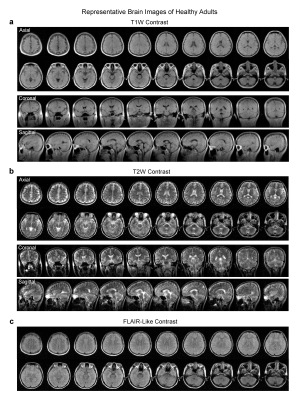
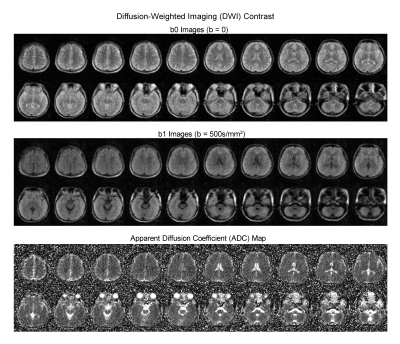
Implementation of four essential protocols: T1W protocol used a 3D GRE sequence with TR/TE=52/13ms, while T2W and FLAIR protocols used a 3D FSE sequence with TR/TE=1500/202ms and 500/129ms respectively. Further, 2D EPI DWI protocol was implemented with TR/TE=2800/102ms and isotropic diffusion weighting factor b-value 500s/mm2. Careful hardware calibration and compensation procedures were implemented. We optimized the protocols for both image SNR and contrast with total scan time ~30min.
Results
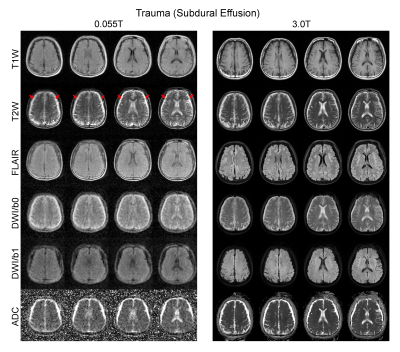
Over 100 subjects were scanned on the 0.055T scanner over a period of 6 months, yielding robust and clinically usable image quality. Figures 2 and 3 show the typical images from healthy adult subjects. Acquisition image resolutions were ~2x2x10mm3 and ~4x4x10mm3 for T1W/T2W/FLAIR and DWI, respectively. Reconstructed image resolution was 1x1x5mm3 for all protocols for better visualization effect.Over 25 subjects were scanned at both 0.055T and 3T (GE Signa Premier). Excellent correspondence was generally observed (see Figure 4 for a trauma patient). The deep learning EMI cancellation method was highly effective in absence of RF shielding cage. It provided a nearly complete removal of EMI noise, with final image noise levels as low as those obtained using the RF shielding cage (within 5% range).
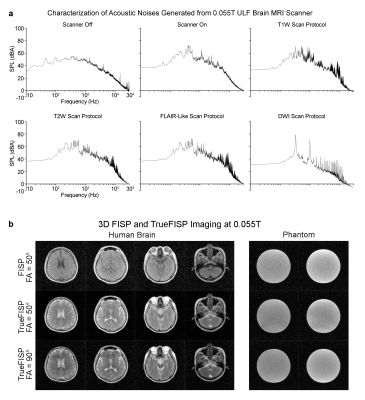
AC power consumption during scanning was low (<1200W). The acoustical noise level was extremely low during scanning (Figure 5A). We also obtained the hardware-demanding TrueFISP images (Figure 5B), indicating future possibility of TrueFISP-based MR fingerprinting12.
Discussion and Conclusions
The high cost of procuring, siting/installing, maintaining and operating the current low-, mid- and high-field clinical scanners constitutes a major roadblock in MRI accessibility in healthcare. Low-cost, low-power, compact, open and shielding-free ULF MRI for brain imaging as demonstrated here aims to complement rather than compete with existing high-performance clinical MRI. ULF MRI holds several inherent attractions, including open magnet configuration for patient comfort, low acoustic scanning noise, low sensitivity to metallic implants, less image susceptibility artifacts, and extremely low RF SAR.In summary, we developed a low cost, shielding-free ULF 0.055T MRI scanner that operated out of a standard AC wall power outlet only. Such scanner can be made low cost to maintain, operate, and manufacture in quantity. This proof-of-concept will catalyze the development of a new class of MRI technologies, together with emerging deep learning methods to reconstruct images from less or noisy data13-15, to democratize MRI for accessible healthcare.
Acknowledgements
This work was supported in part by Hong Kong Research Grant Council (R7003-19F, HKU17112120 and HKU17127121 to E.X.W., and HKU17103819, HKU17104020 and HKU17127021 to A.T.L.L.), and Lam Woo Foundation to E.X.W.References
1. Magnetic resonance imaging (MRI) units (indicator). OECD (2021).
2. Geethanath, S. & Vaughan, J.T., Jr. Accessible magnetic resonance imaging: A review. J Magn Reson Imaging 49, e65-e77 (2019).
3. Marques, J.P., Simonis, F.F.J. & Webb, A.G. Low-field MRI: An MR physics perspective. J Magn Reson Imaging 49, 1528-1542 (2019).
4. O'Reilly, T., Teeuwisse, W.M. & Webb, A.G. Three-dimensional MRI in a homogenous 27cm diameter bore Halbach array magnet. J Magn Reson 307, 106578 (2019).
5. He, Y., He, W., Tan, L., Chen, F., Meng, F., Feng, H. & Xu, Z. Use of 2.1 MHz MRI scanner for brain imaging and its preliminary results in stroke. J Magn Reson 319, 106829 (2020).
6. Sheth, K.N., et al. Assessment of brain injury using portable, low-field magnetic resonance imaging at the bedside of critically ill patients. JAMA Neurol 78, 41-47 (2020).
7. Wald, L.L., McDaniel, P.C., Witzel, T., Stockmann, J.P. & Cooley, C.Z. Low-cost and portable MRI. J Magn Reson Imaging 52, 686-696 (2020).
8. O'Reilly, T., Teeuwisse, W.M., de Gans, D., Koolstra, K. & Webb, A.G. In vivo 3D brain and extremity MRI at 50 mT using a permanent magnet Halbach array. Magn Reson Med 85, 495-505 (2021).
9. Cooley, C.Z., McDaniel, P.C., Stockmann, J.P., Srinivas, S.A., Cauley, S.F., Sliwiak, M., Sappo, C.R., Vaughn, C.F., Guerin, B., Rosen, M.S., Lev, M.H. & Wald, L.L. A portable scanner for magnetic resonance imaging of the brain. Nat Biomed Eng 5, 229-239 (2021).
10. Coffey, A.M., Truong, M.L. & Chekmenev, E.Y. Low-field MRI can be more sensitive than high-field MRI. J Magn Reson 237, 169-174 (2013).
11. Gruber, B., Froeling, M., Leiner, T. & Klomp, D.W.J. RF coils: A practical guide for nonphysicists. J Magn Reson Imaging 48, 590-604 (2018).
12. Ma, D., Gulani, V., Seiberlich, N., Liu, K., Sunshine, J.L., Duerk, J.L. & Griswold, M.A. Magnetic resonance fingerprinting. Nature 495, 187-192 (2013).
13. Zhu, B., Liu, J.Z., Cauley, S.F., Rosen, B.R. & Rosen, M.S. Image reconstruction by domain-transform manifold learning. Nature 555, 487-492 (2018).
14. Antun, V., Renna, F., Poon, C., Adcock, B. & Hansen, A.C. On instabilities of deep learning in image reconstruction and the potential costs of AI. Proc Natl Acad Sci U S A 117, 30088-30095 (2020).
15. Lin, D.J., Johnson, P.M., Knoll, F. & Lui, Y.W. Artificial intelligence for MR image reconstruction: An overview for clinicians. J Magn Reson Imaging 53, 1015-1028 (2021).
Figures