4370
A Feasibility Study of 0.055T MRI for Neuroimaging and Comparison with Clinical 3T MRI1Laboratory of Biomedical Imaging and Signal Processing, The University of Hong Kong, Hong Kong SAR, China, 2Department of Electrical and Electronic Engineering, The University of Hong Kong, Hong Kong SAR, China, 3Department of Diagnostic Radiology, Li Ka Shing Faculty of Medicine, The University of Hong Kong, Hong Kong SAR, China, 4Department of Surgery, Li Ka Shing Faculty of Medicine, The University of Hong Kong, Hong Kong SAR, China, 5Department of Medicine, Li Ka Shing Faculty of Medicine, The University of Hong Kong, Hong Kong SAR, China, 6School of Biomedical Sciences, Li Ka Shing Faculty of Medicine, The University of Hong Kong, Hong Kong SAR, China
Synopsis
Magnetic resonance imaging is a key diagnostic tool in modern healthcare, yet its accessibility is low with the vast majority of clinical MRI scanners being placed in highly specialized radiology departments, large centralized imaging centers, and housed on ground floors of hospitals and clinics. In this study, we deployed our recently developed 0.055T brain ultra-low-field MRI scanner to demonstrate preliminary feasibility in diagnosing tumor and stroke cases with direct comparisons to 3T clinical MRI results. The development of such ULF MRI technologies will enable patient-centric and site-agnostic MRI scanners to fulfill the unmet clinical needs across various global healthcare sites.
Purpose
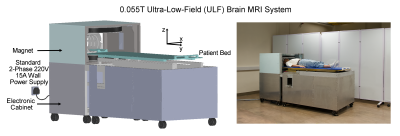
MRI is a key diagnostic tool in modern healthcare1. However, MRI accessibility is low and extremely inhomogeneous worldwide2,3. Presently, the majority of clinical MRI scanners are placed in highly specialized radiology departments and large centralized imaging centers. This excludes easy access for neurology clinics, trauma centers, neonatal/pediatric centers, and community clinics, where clear unmet clinical needs exist. There has been an impetus in developing MRI technologies at ultra-low-field (ULF) strengths3-5 to improve MRI accessibility. Of note is the commercial Hyperfine scanner (www.hyperfine.io/portable-MRI), which has clearly demonstrated clinical potential for point-of-care applications6,7.Recently, we have developed a set of 0.055T brain MRI technologies8. The scanner operates out of the standard AC wall power outlet, with no radiofrequency or magnetic shielding, and is compact and mobile. More importantly, our scanner can implement four universally adopted MRI contrast protocols9-11 for human brain imaging (T1W, T2W, FLAIR like and DWI). Scanning was quiet (≤85dBA) while it was reported that acoustic noise could reach up to 120dBA at 3T12.
In this study, we evaluate the preliminary feasibility for the 0.055T ULF MRI scanner to diagnose several major neurological diseases, including brain tumors, ischemic stroke, and intracerebral hemorrhage (ICH), with direct comparison to clinical 3T MRI results.
Methods
Study was conducted under an institutional review board research protocol approved by The University of Hong Kong/Hospital Authority Hong Kong West Cluster (HKU/HA HKW IRB). A total of 34 out-patients were recruited from neurology and neurosurgery clinics at Queen Mary Hospital, Hong Kong. Six dropped out due to deteriorating medical condition before scheduled scans and a further three were excluded due to chest access issues at the 0.055T scanner. Of the remaining twenty-five patients, thirteen were brain tumor, eight were subacute/chronic stroke and four were subacute/chronic ICH patients. Written informed consent was obtained from all patients prior to any MRI examination and for the publication of images presented here.Patients were examined by 0.055T brain ULF MRI scanner (Figure 1) and a clinical GE 3T MRI scanner (Signa Premier) using standard clinical neuroimaging protocols at Diagnostic Radiology at The University of Hong Kong. Detailed scan protocol parameters can be found in our recent study8. Both 0.055T and 3T images were read on the same day by one senior clinical radiologist, (co-author H.K. Mak). The 0.055T images were read and evaluated first while blinded to the corresponding 3T images.
Results
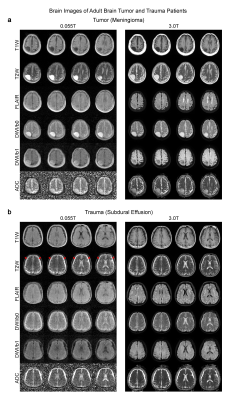
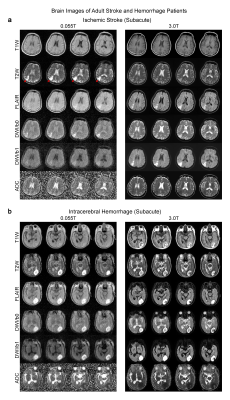
We detected most key pathologies (i.e., the characteristics of signal changes at pathological regions across different contrasts) in 25 patients studied. In a brain tumor case, both 0.055T and 3T images showed a mass in the right parietal cortex that was hypointense in T1W and hyperintense in T2W (Figure 2A). We also showed in a separate patient the ability to detect subdural effusion due to past head trauma at bilateral fronto-parietal regions, showing as a bright signal in 0.055T and 3T T2W images (Figure 2B).In the ischemic stroke case, the 0.055T images were acquired 3 weeks after the 3T MRI scan conducted two days after index stroke. The ischemic infarct in the right parietal cortex was mildly hyperintense in 0.055T DWI and hyperintense in 3T DWI, while ADC showed slight hyperintensity at 0.055T and hypointensity at 3T (Figure 3A). These results are highly consistent with the expected progression of ischemic stroke across different timepoints9 (i.e., subacute vs. acute). In a subacute ICH case, a hematoma was identified at the left occipital lobe in both 0.055T and 3T images (Figure 3B). The hematoma exhibited a hyperintense rim with a hypointense core in T2W images, indicating blood product and/or hemosiderin of different stages at the rim and core regions.
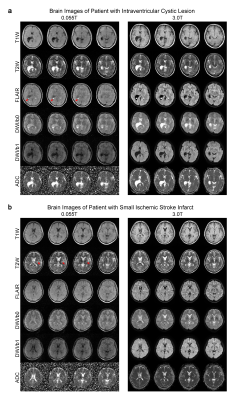
We also demonstrated the ability to detect rare intraventricular cystic lesions. 0.055T FLAIR contrast exhibited excellent sensitivity and correspondence with 3T results in detecting a choroid plexus cyst (i.e., hyperintense signal) located at the occipital horn of the right lateral ventricle (Figure 4A). Further, we could also observe small lacunar infarcts (~3mm) in 0.055T images, not just large (>10mm) infarcts, in a separate chronic stroke case (Figure 4B).
Discussion and Conclusions
In this study, we succeeded in demonstrating the potential clinical utility of our 0.055T MRI in diagnosing tumor and stroke with direct comparisons with clinical 3T results. Further, we showed that the presence of paramagnetic (i.e., titanium and titanium alloys) and ferromagnetic (i.e., cobalt, nickel and associated alloys) materials in aneurysm clips and cerebrovascular stents did not induce gross artifacts in the 0.055T images (Figure 5). We expect that ULF will enable MRI scanning of patients with medical metal implants or accident-related metal fragments, who would otherwise not be candidates for conventional high-field MRI. Overall, ULF MRI holds several inherent attractions when compared to high-field MRI. They include open magnet configuration for patient comfort, low acoustic noise levels during scanning, low sensitivity to metallic implants, less image susceptibility artifacts at air/tissue interfaces, and extremely low RF specific absorption rate. Together with complete elimination of RF and magnetic shielding cages, our ULF MRI approach can offer a more patient-friendly alternative to complement existing high-performance clinical MRI and fulfill the unmet clinical needs across various healthcare communities.Acknowledgements
This work was supported in part by Hong Kong Research Grant Council (R7003-19F, HKU17112120 and HKU17127121 to E.X.W., and HKU17103819, HKU17104020 and HKU17127021 to A.T.L.L.), and Lam Woo Foundation to E.X.W.References
1. Fuchs, V.R. & Sox, H.C., Jr. Physicians' views of the relative importance of thirty medical innovations. Health Aff (Millwood) 20, 30-42 (2001).
2. Magnetic resonance imaging (MRI) units (indicator). OECD (2021).
3. Geethanath, S. & Vaughan, J.T., Jr. Accessible magnetic resonance imaging: A review. J Magn Reson Imaging 49, e65-e77 (2019).
4. Marques, J.P., Simonis, F.F.J. & Webb, A.G. Low-field MRI: An MR physics perspective. J Magn Reson Imaging 49, 1528-1542 (2019).
5. Wald, L.L., McDaniel, P.C., Witzel, T., Stockmann, J.P. & Cooley, C.Z. Low-cost and portable MRI. J Magn Reson Imaging 52, 686-696 (2020).
6. Sheth, K.N., et al. Assessment of brain injury using portable, low-Field magnetic resonance imaging at the bedside of critically ill patients. JAMA Neurol 78, 41-47 (2020).
7. Mazurek, M.H., et al. Portable, bedside, low-field magnetic resonance imaging for evaluation of intracerebral hemorrhage. Nat Commun 12, 5119 (2021).
8. Liu, Y., Leong, A.T.L., Zhao, Y., Xiao, L., Mak, H.K.F., Tsang, A., Lau, G.K.K., Leung, G.K.K. & Wu, E.X. A low-cost and shielding-free ultra-low-field brain MRI scanner. Nat Commun in press, MS#28454 (2021).
9. Merino, J.G. & Warach, S. Imaging of acute stroke. Nat Rev Neurol 6, 560-571 (2010).
10. Asdaghi, N. & Coutts, S.B. Stroke: neuroimaging in acute stroke-where does MRI fit in? Nat Rev Neurol 7, 6-7 (2011).
11. Chalela, J.A., Kidwell, C.S., Nentwich, L.M., Luby, M., Butman, J.A., Demchuk, A.M., Hill, M.D., Patronas, N., Latour, L. & Warach, S. Magnetic resonance imaging and computed tomography in emergency assessment of patients with suspected acute stroke: a prospective comparison. Lancet 369, 293-298 (2007).
12. Jin, C., Li, H., Li, X., Wang, M., Liu, C., Guo, J. & Yang, J. Temporary hearing threshold shift in healthy volunteers with hearing protection caused by acoustic noise exposure during 3-T multisequence MR neuroimaging. Radiology 286, 602-608 (2018).
Figures