4366
Hyperpolarized 13C-pyruvate MR metabolic imaging in Aging1Department of Biomedical Science, Chonnam National University, Gwangju, Korea, Republic of, 2Department of Artificial Intelligence Convergence, Chonnam National University, Gwangju, Korea, Republic of, 3Department of Internal Medicine, Chonnam National University, Gwangju, Korea, Republic of, 4Department of Radiology, Chonnam National University, Gwangju, Korea, Republic of
Synopsis
Hyperpolarized [1-13C1]pyruvate MRI data were obtained in vivo metabolic data from young and aged animals in rodent to elucidate the different changes in metabolism due to aging. The preliminary results in this study demonstrated the feasibility of using hyperpolarized MRI 13C technique for identifying renal metabolic changes between aged and young animals. The reduction of Lac/Pyr, Ala/Pyr and BiC/Pyr ratio may be used as biomarkers for the cellular dysfunction that accompanies the aging process. The results from this study warrant further research on the usage of hyperpolarized 13C MRI in monitoring metabolic changes in aging-related studies.
INTRODUCTION
Research on aging focuses on expanding our knowledge about the underlying mechanism of cellular damage which results in progressive loss of organ function [1]. Energy metabolism can be a potential biomarker of aging, which provides information about the aging process and aging-associated disease. With the recent development of dynamic nuclear polarization, hyperpolarized magnetic resonance imaging (HP-MRI) enhances signal sensitivity over 10,000 fold compared to the conventional technique. Following the injection of hyperpolarized 13C substrates, the real-time HP-MRI enables the noninvasive measurement of in vivo metabolic activity. In this study, we utilized hyperpolarized [1-13C1]pyruvate MR metabolic imaging to obtain in vivo metabolic data from young and aged animals in rodent and sought to elucidate the different changes in metabolism due to aging.METHODS
All study procedures were approved by the Institutional Animal Care and Use Committee. Male Fischer 334 (F334) rats were purchased and raised with a normal diet. The animals were divided into 2 group: young (n = 9; mean age, 1.8 months; range, 1.7-2.0 months) and aged (n = 5; mean age, 25.3 months; range, 24.7-26.6 months). The animals were fasted for 2 hours prior to the MRI scans and all imaging exams were performed at approximately the same time of the day, in order to minimize any physiological variations.The HP-MRI data were obtained using a GE clinical 3T scanner with a custom 13C/1H dual rat coil. 32mL of pyruvate was polarized using a HyperSense DNP polarizer. High resolution axial T2-weighted images were acquired for anatomical reference. The following HP-MRI data were acquired after the injection of 3mL HP [1-13C1]pyruvate solution (100 mM and pH of 7.5) through tail vein: 1) Slice-localized dynamic 13C MR spectroscopy (MRS) data (TE/TR=35/3000ms, flip angle=20, 3s temporal resolution, 60 total time points) from 40mm slab centered in kidney, 2) compressed sensing 3D 13C magnetic resonance spectroscopic imaging (MRSI) data [2]. The hyperpolarized substrate and its metabolic products were compared between the young and old animals using an unpaired t-test.
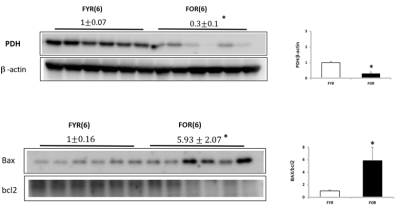
At the completion of the imaging, the kidneys were resected and the following biological tests were performed: western blot of pyruvate dehydrogenase (PDH), Bax, and Bcl2.
RESULTS
Figure 1 illustrates representative 3D 13C MRSI data from a kidney of a young rat. The sagittal and axial image of the mouse show the 5.4 mm slice that the data were acquired from and the spatially-resolved 13C spectroscopic imaging voxels overlaid on top of the axial image. The yellow voxels indicate the location of the 13C kidney data that were used for metabolite quantification. Figure 2 shows one example of slice-localized dynamic 13C MRS data in the kidney of a young rat.The quantifications of the HP 13C 3D MRSI and slice-localized dynamic 13C MRS data are summarized in Figure 3 and 4, respectively. In the 3D MRSI data, both the ratio of lactate-to-pyruvate (Lac/Pyr) and alanine-to-pyruvate (Ala/Pyr) demonstrated significantly lower levels in the aged group (0.08 ± 0.016 for Lac/Pyr and 0.05 ± 0.015 for Ala/Pyr) compared to those in the young group (0.17 ± 0.042 for Lac/Pyr, p = 0.003 and 0.13 ± 0.040 for Ala/Pyr, p = 0.003). In the dynamic data, the AUC ratios of Ala/Pyr and bicarbonate-to-pyruvate (Bic/Pyr) in the aged rats (0.11 ± 0.008 for Ala/Pyr and 0.04 ± 0.003 for Bic/Pyr) were significantly lower than those in the young rats (0.14 ± 0.014 for Ala/Pyr, p = 0.001 and 0.046 ± 0.004 for Bic/Pyrp = 0.003). The decreased level of bicarbonate in the aged animals was consistent with the reduction in PDH level of old rats compared to young ones (Fig5). In addition, the elevated level of Bax/Bcl2 ratio was observed in the aged animal.
DISSCUSSION
We found that the levels of lactate, alanine and bicarbonate were significantly reduced in the aged group, which suggests the systematic reduction in in vivo metabolic activity. These systemic reduction in metabolism may indicate a sign of cellular damage and mitochondrial dysfunction in kidney, which is one of the nine hallmarks of aging. In agreement, the abnormality in mitochondrial function was observed by the decrease in PDH level [3] and the increase in Bax/Bcl2 level, which is related to the high rate of apoptosis, another well-known characteristic of aging [4].CONCLUSION
The preliminary results in this study demonstrated the feasibility of using hyperpolarized MRI 13C technique for identifying renal metabolic changes between aged and young animals. The reduction of Lac/Pyr, Ala/Pyr and Bic/Pyr ratio may be used as biomarkers for the cellular dysfunction that accompanies the aging process. The results from this study warrant further research on the usage of hyperpolarized 13C MRI in monitoring metabolic changes in aging-related studies.Acknowledgements
This study was supported by the Ministry of Education, Republic of Korea (2019R1I1A3A01059201).
References
1. Lopez-Otin, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The hallmarks of aging. Cell 2013, 153, 1194-1217, doi:10.1016/j.cell.2013.05.039.
2. Park I, et al. Evaluation of heterogeneous metabolic profile in an orthotopic human glioblastoma xenograft model using compressed sensing hyperpolarized 3D 13C magnetic resonance spectroscopic imaging. Magn Reson Med. 2012;70(1):33-9.
3. Stacpoole, P.W. The pyruvate dehydrogenase complex as a therapeutic target for age-related diseases. Aging Cell 2012, 11, 371-377, doi:10.1111/j.1474-9726.2012.00805.x.
4. Raisova, M.; Hossini, A.M.; Eberle, J.; Riebeling, C.; Wieder, T.; Sturm, I.; Daniel, P.T.; Orfanos, C.E.; Geilen, C.C. The Bax/Bcl-2 ratio determines the susceptibility of human melanoma cells to CD95/Fas-mediated apoptosis. J Invest Dermatol 2001, 117, 333-340, doi:10.1046/j.0022-202x.2001.01409.x.
Figures
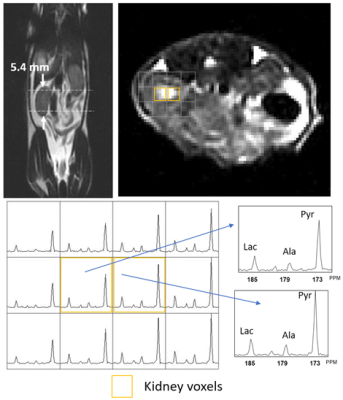
Figure 1. Representative 3D MRSI data, showing sagittal and axial T2 MRI of kidney and the corresponding 13C spectra in young animal.
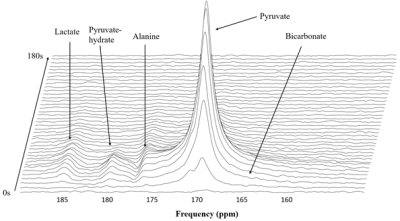
Figure 2. Representative dynamic hyperpolarized 13C data acquire from a 40mm slab around kidney.
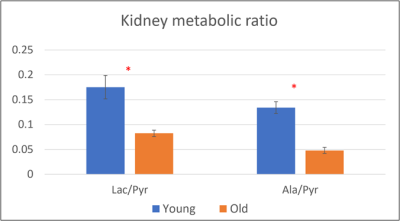
Figure 3. The quantification of hyperpolarized 13C 3D MRSI experiment (*, p < 0.05)
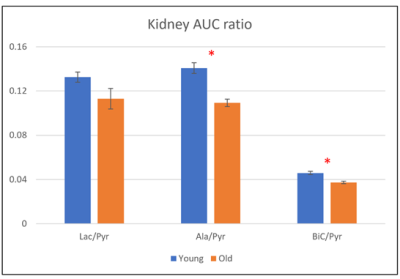
Figure 4. The quantification of slice-localized dynamic hyperpolarized 13C experiment (*, p < 0.05)