4364
Echo time optimization for in-vivo measurement of unsaturated lipid protons using J-difference-edited MRS.1College of Biomedical Engineering & Instrument Science, Zhejiang University, Hangzhou, China, 2MR Collaboration, Siemens healthineers ltd., Shanghai, China, 3Department of Endocrine, Sir Run Run Shaw Hospital, Hangzhou, China
Synopsis
In this study, J-difference editing was applied to unsaturated lipid protons. Density-matrix simulations were devised to demonstrate the TE-dependent signal evolution of J-coupled protons. Phantom and in-vivo experiments on human muscles were performed to verify the simulation results. The optimal TE was determined as 45 ms with the signals gain of 148.03% for allylic and 2.37% for diallylic groups on phantom and 160.16% for allylic and 13.22% for diallylic groups on human muscles, when compared to TE of 70 ms. This edited-MRS protocol allows robust quantification of unsaturated lipid composition in-vivo and investigation on lipid metabolism in future.
Introduction
Compared to saturated fatty acids, mono-unsaturated fatty acids and poly-unsaturated fatty acids have different chemical structures and characteristics in metabolic pathways1. Whereas each of them plays a unique role in lipid metabolism, the changes of lipid composition may affect many important signaling pathways in vivo, including the building up of insulin resistance2. Lipid composition can be detected noninvasively by conventional proton magnetic resonance spectroscopy (1H MRS)3. However, the discovery of unsaturated lipid signal on regular MR spectra is particularly hard due to the relatively low abundance of unsaturated groups and the complexity of their peak shape4. The J-difference-edited (JDE) approach offers the opportunity to selectively detect the unsaturated lipid protons and calculate the lipid composition in vivo without the contamination from the overlapping signals of residue water and other molecules5. This study was to exploit the JDE method and to optimize the protocol for best resolving and quantification of unsaturated lipid in vivo. The TE efficacy of JDE protocol was verified in simulation, oil phantom and in-vivo experiments at 3T.Methods
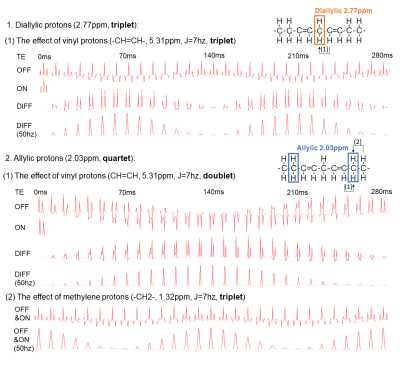
Instruments: Experiments were conducted on Siemens MAGNETOM Prisma 3T MRI scanners using 18-channel body coils.Simulations: Density-matrix simulations were performed for B0 = 2.89T using NMR-Scope in jMRUI-v4. The diallylic group was simulated considering only the coupling between the vinyl and diallylic protons with a MEGA-PRESS sequence6, while for the allylic group, coupling between vinyl, allylic and methylene protons were all considered in the simulation (Figure 1a). The signal amplitudes were measured by AMARES algorithm and T2 attenuation for all lipid protons were considered using the T2 value of 76 ms7.
Phantom Experiments: A 1-Liter soybean oil phantom was prepared. JDE experiments were performed using MEGA-PRESS sequence at TEs of 40-80 ms in 5 ms increments. The scan parameters included: TR = 2000 ms; spectral width = 2 kHz; voxel size = 40 mm3; 8 transients and 2048 datapoints. The edited-pulse bandwidth was 150 Hz and the edited frequency was 5.31 ppm (editing off at 7.5 ppm).
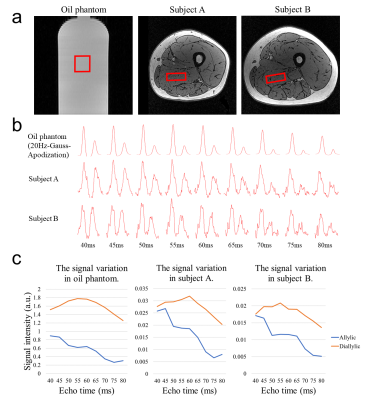
In-vivo Experiments: Two healthy volunteers (N=2, male, 23/24 years-old) were recruited. A 47*40*17 mm3 MRS voxel was located within adductor magnus using VAPOR water suppression (Figure 4a). Measurements were performed using the same acquisition parameters as the phantom experiments except 32 transients were acquired.
Data Processing: Automatic spectral registration was performed with the FID-A toolbox using the methyl and bulk methylene signal as the landmark. AMARES was used to quantify allylic and diallylic resonances. For the quantification in human muscles, both intra-myocellular lipid (IMCL) and extra-myocellular lipid (EMCL) components were fitted independently.
Results
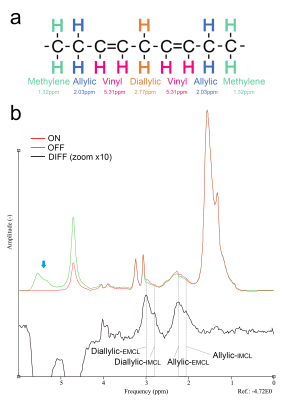
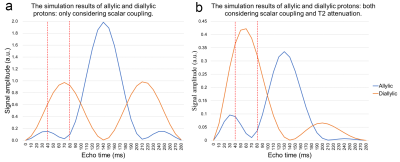
Figure 1b shows the JDE spectra from skeletal muscle in vivo. When selectively editing the vinyl protons at 5.31 ppm in two transients (ON/OFF), the allylic protons and the diallylic protons can be detected in the difference spectra (DIFF).Figure 2 shows the simulation of signal evolution of allylic and diallylic protons as a function of TE without T2 attenuation. The maximum peak amplitude of diallylic is reached at TE equals 70 ms (~1/(2J)) or 210 ms (~3/(2J)), while allylic is reached at TE equals 140 ms (~1/J).
Figure 3a summarizes the peak amplitude change of these two types of unsaturated lipid protons and figure 3b presents the peak amplitude change incorporated T2 attenuation effect. From TE of 40 to 80 ms, signal allylic shows a local minimum at 70 ms while diallylic reaches its maximum at 60 ms.
Figure 4 exhibits the change of signals with different TEs in oil phantom and human muscles. Allylic signals showed a decreasing trend and reached the minimum around TE of 75~80 ms, yet diallylic signals reached the maximum around 55~60 ms, which is consistent with the simulation.
Discussion
The detection and quantification of the characteristic signal of unsaturated lipid is of high importance. With JDE MRS, the major methylene signals (1.32 ppm), water signal (4.7 ppm) and other molecules in tissue were not coupled with vinyl groups and hence will not be included in the difference spectra. The detection of allylic and diallylic protons is free of contaminations. Lindeboom et al.5 were the first to present a successful lipid editing experiment on human muscles with TE of 70 ms. However, the diallylic signal is relatively low compared to allylic. Combining the simulation and phantom experiments, we found that allylic and diallylic signals are unable to reach the maximum simultaneously when considering both J-coupling evolution and T2 relaxation8. To maximize the detection of both allylic and diallylic signals, TE of 45 ms is suggested to be the optimal. While the signal gain at TE of 45 ms has been verified as 148.03% for allylic and 2.37% for diallylic protons on oil phantom and 160.16% for allylic and 13.22% for diallylic protons on human muscles (mean values) compared to TE of 70 ms.Conclusion
The simulations of unsaturated lipid proton signals have been verified by phantom and in-vivo experiments, suggesting edited MRS on unsaturated lipid protons using TE of 45 ms is optimal, which allows stable and robust in-vivo quantification of different unsaturated lipid molecules and facilitates the clinical investigation of unsaturated lipid metabolism in future.Acknowledgements
No acknowledgement found.References
1. Tvrzicka E, Kremmyda LS, Stankova B, Zak A. Fatty acids as biocompounds: their role in human metabolism, health and disease--a review. Part 1: classification, dietary sources and biological functions. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub 2011;155(2):117-130.
2. Corcoran MP, Lamon-Fava S, Fielding RA. Skeletal muscle lipid deposition and insulin resistance: effect of dietary fatty acids and exercise. Am J Clin Nutr 2007;85(3):662-677.
3. Fallone CJ, Tessier AG, Field CJ, Yahya A. Resolving the omega-3 methyl resonance with long echo time magnetic resonance spectroscopy in mouse adipose tissue at 9.4 T. NMR Biomed 2021;34(2):e4455.
4. Haus JM, Solomon TP, Kelly KR, Fealy CE, Kullman EL, Scelsi AR, Lu L, Pagadala MR, McCullough AJ, Flask CA, Kirwan JP. Improved hepatic lipid composition following short-term exercise in nonalcoholic fatty liver disease. J Clin Endocrinol Metab 2013;98(7):E1181-1188.
5. Lindeboom L, de Graaf RA. Measurement of lipid composition in human skeletal muscle and adipose tissue with (1) H-MRS homonuclear spectral editing. Magn Reson Med 2018;79(2):619-627.
6. Stefan D, Cesare FD, Andrasescu A, Popa E, Lazariev A, Vescovo E, Strbak O, Williams S, Starcuk Z, Cabanas M, van Ormondt D, Graveron-Demilly D. Quantitation of magnetic resonance spectroscopy signals: the jMRUI software package. Measurement Science and Technology 2009;20(10).
7. Gajdosik M, Hingerl L, Skoch A, Freudenthaler A, Krumpolec P, Ukropec J, Ukropcova B, Sedivy P, Hajek M, Itariu BK, Maier B, Baumgartner-Parzer S, Krebs M, Trattnig S, Krssak M. Ultralong TE In Vivo (1) H MR Spectroscopy of Omega-3 Fatty Acids in Subcutaneous Adipose Tissue at 7 T. J Magn Reson Imaging 2019;50(1):71-82.
8. Thankamony A, Kemp GJ, Koulman A, Bokii V, Savage DB, Boesch C, Hodson L, Dunger DB, Sleigh A. Compositional marker in vivo reveals intramyocellular lipid turnover during fasting-induced lipolysis. Sci Rep 2018;8(1):2750.
Figures