4359
Assessment of Glx and GABA levels in the Global Developmental Disability Children: A Magnetic Resonance Spectroscopy Study1Radiology, the First Hospital of Jilin University, Changchun, China, 2Philips Healthcare, Beijing, China, Beijing, China, 3Departments of Developmental Behavioral Pediatrics, the First Hospital of Jilin University, Changchun, China
Synopsis
This study aims to explore the correlation between brain neuro metabolites and clinical symptom scales in GDD and GDD+ASD. 11 children with GDD and 12 GDD +ASD children took MR scans, and completed the GABA and Glx spectroscopic acquisition. Results indicated that there is a significant difference in DQ language levels between GDD and GDD+ASD, and the GABA/Cr levels were significantly decreased in GDD+ASD compared with GDD. A positive correlation was observed between Glx/Cr and ADOS stereotype among all participants, and a clear link was observed between Glx/Cr and the sensory symptoms of ASD at both behavioral and perceptual levels.
Introduction
The clinical manifestations and etiology of global developmental disabilities (GDD) and the autism spectrum disorder (ASD) overlap somewhat, and common comorbidity occurs[1-3]. Patients with ASD and/or GDD have neurodevelopmental disorders that can eventually cause neurological damage. The E/I balance neurotransmitters plays an important role in maintain of normal brain function, otherwise, multiple neurological or psychiatric disorders may occur. Hussman et al.[4] proposed the neurotransmitter imbalance theory of ASD, where they found that the upregulation of the excitatory neurotransmitter glutamate (Glu) to the inhibitory neurotransmitter gamma-aminobutyric acid (GABA) ratio was closely correlated with the onset of GDD and ASD. However, the concentration of GABA and Glu in the brain is too low to be reliably detected using conventional 1H magnetic resonance spectroscopy (MRS). The edited MRS, e.g. Mescher-Garwood Point-resolved Spectroscopy (MEGA-PRESS), was thus developed for specific detection of metabolites including GABA [5,6] and Glu. This study aims to detect the alterations and explore the correlations of brain neuro metabolites including GABA and glutamate-glutamine (Glx) with clinical symptoms in GDD and GDD+ASD by using MEGA-PRESS.Materials and methods
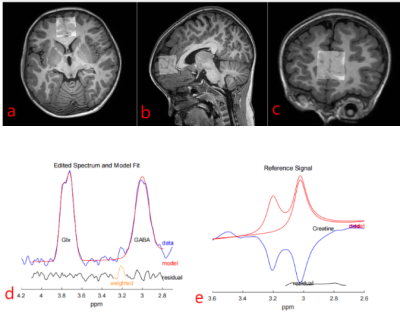
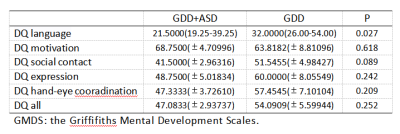
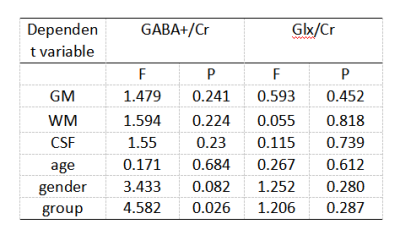
11 children with GDD (7 male, aged 27-48 months), 12 GDD +ASD children (8 male, aged 11-65 months) completed the MEGA-PRESS acquisition. The participants in the GDD+ASD group were diagnosed based on the DSM-IV-TR criteria and were assessed by ADOS-2[7]. GDD group was diagnosed with mental retardation or communication disorders according to the DSMIV-TR criteria and ADOS-2. All participants were assessed by the Chinese version of the Griffifiths Mental Development Scales (GMDS) and the infant-junior high school social life ability scale (SM). MR scans were carried out on a 3.0T scanner (Ingenia Elition, Philips Healthcare, Best, the Netherlands) with a 32-channel head coil. Parameters for MEGA-PRESSS were as follows: TR/TE 2000/68 ms, voxel size 3×3×3 cm3, about 10 min per acquisition. The regions of interest (ROIs) for MEGA-PRESSS were set at ventromedial prefrontal cortex (VMPFC). The detected GABA and Glx signals in GDD and ASD+GDD were quantified using the Matlab-based (MathWorks, Natick, MA) analysis toolkit Gannet 3.0 (http://www.gabamrs.com/) with the creatine (Cr) signal as an internal reference (Figure 1). The GABA signal detected by MEGA-PRESS also contains co-edited signal from macromolecules and homocarnosine, so it is referred to as GABA+ below. Only spectra with a relative fitting error (FitError) of GABA+/Cr and Glx/Cr generated by Gannet smaller than 15% were enrolled in the final statistical analysis. Differences of GABA+ and Glx levels between the two groups were analyzed using t test and analysis of covariance (ANOVA, adjusting for GM, WM and CSF fractions [8]).Results
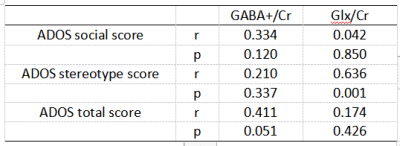
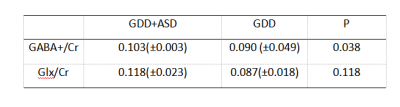
The Mann-whitney U test results indicated that there is a significant difference in DQ language levels between GDD and GDD+ASD (U=2.218, p=0.027), while no difference was found in DQ motivation, DQ hand-eye coordination, DQ social contact, DQ expression, DQ all and SM, as indicated in Table1. A positive correlation was observed between the Glx/Cr level and the ADOS stereotype score in all participants (r = 0.636 p = 0.001, Table 2). A significant difference in GABA levels was observed between GDD and GDD+ASD groups (F=3.188, p=0.038), while no difference was found in Glx levels, with effects of GM, WM, CSF and age (Tables 3-4).Discussions
VMPFC plays an important role in regulating emotion and behavior , neurons within the VMPFC mainly include pyramidal efferent neurons and GABA-ergic interneurons[9,10]. In patients with ASD, abnormal levels and function of GABA were detected by serum and brain signals, the reduced density of GABA-A and GABA-B receptors in VMPFC were associated with ASD symptom severity[11,12]. The diminished valuation-related brain responses in VMPFC to stimuli that portend social interaction may contribute to deficits in social exchange observed in individuals diagnosed with ASD. At present, a large body of research evidence suggests a reduced GABA activity in the brain of ASD patients, our research is consistent with this view.The E-I balance theory of GDD+ASD and GDD consider excitatory glutamatergic and inhibitory GABAergic signaling as independent processes. The measurement of glutamate in this study was contaminated with glutamine, and glutamine also functions as a precursor for the biosynthesis of GABA via glutamate, meaning that some of the Glx being measured represents glutamine and glutamate that function as precursors to GABA [13,14]. Elevated Glx levels, which is indirectly indicative of increased glutamatergic signaling, may explain greater difficulties with sensory discrimination and more difficulties with sensory gating, which could account for the results.Although altered E-I balance has long been thought to underlie sensory symptoms of GDD and ASD, a clear association between E/I markers and sensory symptoms of ASD has been lacking. Jason[15] took a comprehensive approach to linking E/I to sensory symptoms, measuring both GABA and Glu levels as markers of the E/I balance, as well as assessing sensory symptoms at both behavioral and perceptual levels. Our findings provide a strong evidence in support of the altered E-I balance theory of ASD.Summary of Findings
A lower GABA+ level was observed in VMPFC in children with GDD+ASD than with GDD, and a clear link was observed between the Glx level and the sensory symptoms of ASD at both behavioral and perceptual levels.Acknowledgements
No acknowledgement found.References
1. Maenner MJ, Shaw KA, Baio J, et al. Prevalence of Autism Spectrum Disorder Among Children Aged 8 Years - Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2016. MMWR Surveill Summ. 2020;69(4):1‐12.2. Coe BP, Stessman HAF, Sulovari A, Geisheker MR, Bakken TE, Lake AM, Dougherty JD, Lein ES, Hormozdiari F, Bernier RA, Eichler EE. Neurodevelopmental disease genes implicated by de novo mutation and copy number variation morbidity. Nat Genet. 2019 Jan;51(1):106-116.3. Miller LE, Burke JD, Robins DL, Fein DA. Diagnosing Autism Spectrum Disorder in Children with Low Mental Age. J Autism Dev Disord. 2019 Mar;49(3):1080-1095.4. Hussman JP. Suppressed GABAergic inhibition as a common factor in suspected etiologies of autism. J Autism Dev Disord. 2001;31(2), 247-248.5. Gong T, Xiang Y, Saleh MG et al (2018) Inhibitory motor dysfunction in parkinson's disease subtypes. J Magn Reson Imaging 47:1610-1615.6. Mescher M, Merkle H, Kirsch J, Garwood M, Gruetter R (1998) Simultaneous in vivo spectral editing and water suppression. NMR Biomed 11:266-272.7. Schopler E, Reichler RJ, Renner BR (1986) Toward objective classification of childhood autism – childhood autism ratingscale (CARS). J Autism Dev Disord 10(1):91–103.8. Chowdhury FA, O'Gorman RL, Nashef L et al (2015) Investigation of glutamine and GABA levels in patients with idiopathic generalized epilepsy using MEGAPRESS. J Magn Reson Imaging 41:694-699.9. Koenigsa M, Grafman J. The functional neuroanatomy of depression: Distinct roles for ventromedial and dorsolateral prefrontal cortex. Behavioural Brain Research.2009(201).10. Antoine MW, Langberg T, Schnepel P, Feldman DE. Increased Excitation-Inhibition Ratio Stabilizes Synapse and Circuit Excitability in Four Autism Mouse Models. Neuron. 2019 Feb 20;101(4):648-661.e4.11. Rojas DC, Singel D, Steinmetz S, et al. Decreased left perisylvian GABA concentration in children with autism and unaffected siblings. Neuroimage. 2014;86,28-34.12. Cochran DM, Sikoglu EM, Hodge SM, et al. Relationship among glutamine, γ-aminobutyric acid, and social cognition in autism spectrum disorders. J Child Adolesc Psychopharmacol. 2015;25,314-322.13. Willard SS, Koochekpour S. Glutamate, glutamate receptors, and downstream signaling pathways. Int J Biol Sci. 2013;9:948-59.14. Javitt DC, Schoepp D, Kalivas PW, Volkow ND, Zarate C, Merchant K, et al. Translating glutamate: from pathophysiology to treatment. Sci Transl Med. 2011;3:10215. Jason L,Georg O,Mark M, et al. Region-specific elevations of glutamate + glutamine correlate with the sensory symptoms of autism spectrum disorders.Translational Psychiatry (2021) 11:411.
Figures