4354
Real-time High-quality Multi-parametric 4D-MRI Using Deep Learning-based Motion Estimation from Ultra-undersampled Radial K-space1Health Technology and Informatics, The Hong Kong Polytechnic University, Hong Kong, China, 2Key Laboratory of Carcinogenesis and Translational Research (Ministry of Education/Beijing), Department of Radiation Oncology, Beijing Cancer Hospital & Institute, Peking University Cancer Hospital & Institute, Beijing, China, 3Department of Clinical Oncology, The University of Hong Kong, Hong Kong, China, 4Department of Clinical Oncology, Queen Mary Hospital, Hong Kong, China, 5Department of Radiology, The University of Hong Kong, Hong Kong, China
Synopsis
We have developed and validated a deep learning-based real-time high-quality (HQ) multi-parametric (Mp) 4D-MRI technique. A dual-supervised downsampling-invariant deformable registration (D3R) model was trained on retrospectively downsampled 4D-MRI with 100 radial spokes in the k-space. The deformations obtained from the downsampled 4D-MRI were applied to 3D-MRI to reconstruct HQ Mp 4D-MRI. The D3R model provides accurate and stable registration performance at up to 500 times downsampling, and the HQ Mp 4D-MRI shows significantly improved quality with sub-voxel level motion accuracy. This technique provides HQ Mp 4D-MRI within 500 ms and holds great potential in online tumor tracking in MR-guided radiotherapy.
Introduction
Accurate radiation beam delivery is vital in radiation therapy (RT), especially for treatment techniques with large dose fractions, such as stereotactic body radiation therapy (SBRT). Real-time tumor motion tracking is an emerging technique for monitoring radiation delivery, and its benefits on radiation dosimetry have been proved1. In magnetic resonance-guided Radiotherapy (MRgRT), current onboard imaging relies on single or orthogonal two-dimensional (2D) cine magnetic resonance imaging (MRI)2, which only provides T1-weighted (T1w) image contrast and incomplete tumor motion. Multi-parametric (Mp) four-dimensional (4D) MRI provides versatile tumor contrasts and accurate tumor motion; however, the imaging time is prohibitively long for real-time application3. According to the American Association of Physicists in Medicine (AAPM) task group report, real-time tumor tracking requires the total latency between a respiratory motion and dose delivery to be less than 500 ms4, allowing an even shorter time for image acquisition and reconstruction. This study developed a deep learning-based dual-supervised downsampling-invariant deformable registration (D3R) model for motion estimation from ultra-undersampled radial k-space and reconstructed high-quality (HQ) Mp 4D-MRI in real-time. Our results showed that the D3R model is robust to incomplete k-space data up to a downsampling factor of 500. The real-time HQ Mp 4D-MRI accurately reflects the tumor motion trajectory with sub-voxel level accuracy.Methods
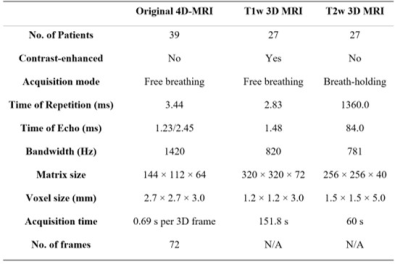
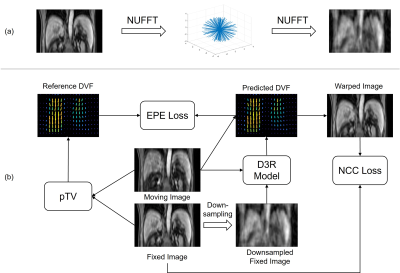
Thirty-nine patients were collected from Beijing Cancer Hospital with institutional review board approval. Each patient underwent a T1w 4D-MRI using TWIST volumetric interpolated breath-hold examination (TWIST-VIBE) sequence5. Of the thirty-nine patients, twenty-seven also underwent regular T1w and T2-weighted (T2w) 3D MRI scans. Details of the imaging parameters are listed in Table 1. For D3R model training, the original 4D-MRI were sorted into 10 phase bins based on the body area6. After data cleaning, the training and validation sets comprised 120 image pairs from 24 patients, and the testing set comprised 15 image pairs from 3 patients. Image binning was not needed for later HQ Mp 4D-MRI reconstruction, as deformations were directly estimated on the time-resolved original 4D-MRI.Figure 1 illustrates the data preparation and D3R model training processes. As shown in Figure 1a, the original 4D-MRI images were retrospectively down-sampled using non-uniform fast Fourier transform (NUFFT) in 3D radial read-out. Typical linear accelerator response time is 80 ms7, and GPU-based 3D NUFFT reconstruction takes 50 ms, leaving around 300 ms for image acquisition. Since typical time of repetition (TR) in the T1w MRI sequence is around 3 ms, 100 radial spokes were utilized to reconstruct the training images. To evaluate the robustness of the D3R model, test images with 25, 50, 100, 150, 200, and 400 radial spokes were also included. The downsampling factor was defined as R=(Mx×My×π/2)/NSP, where NSP is the number of radial spokes and Mx and My are matrix size in x and y directions, respectively. Figure 1b shows the D3R model training process, which was based on 3D U-Net8. Reference deformation vector fields (DVFs) were calculated between the original image pairs using parametric total variation (pTV) algorithm. The training was dually supervised by the end-to-end point error (EPE) between reference DVFs and predicted DVFs and the normalized cross-correlation (NCC) between warped images and fixed images. In testing, both EPE and image similarity metrics, including structure similarity index (SSIM), peak signal-to-noise ratio (PSNR), and mean squared error (MSE), were calculated between the warped and fixed images. Conventional methods such as Elastix, Demons, and pTV algorithms were included in the performance evaluation for comparison.
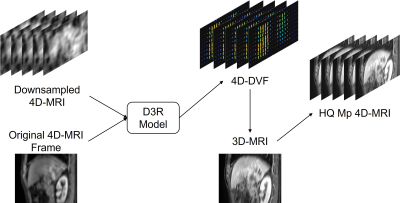
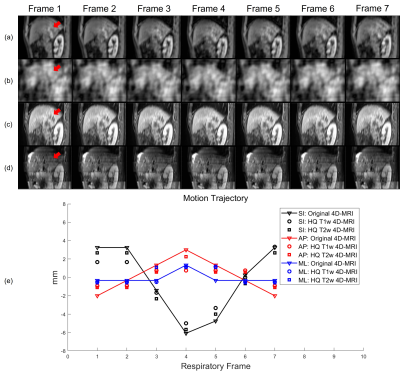
Figure 2 illustrates the HQ Mp 4D-MRI reconstruction process. 4D-DVF obtained from downsampled 4D-MRI via D3R model was applied to the pre-aligned 3D MRI to reconstruct HQ Mp 4D-MRI at corresponding frames. For motion accuracy validation, tumor trajectories were tracked and compared both in the original 4D-MRI and reconstructed HQ Mp 4D-MRI.
Results
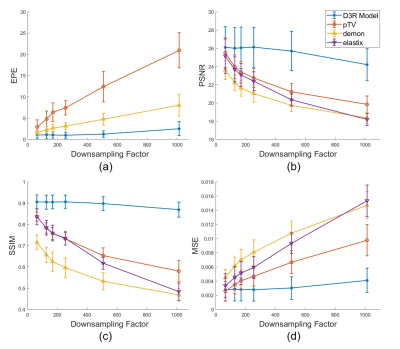
Figure 3 plots the evaluation metrics versus downsampling factor R. As shown in Figure 3a, the Demons and pTV algorithms crashed and gave increasingly different DVFs compared to those calculated on the original images as R becomes larger. Figure 3b, 3c, and 3d demonstrate similar results, in which the image similarity drops fast as R grows. By contrast, the proposed D3R model gives consistent DVFs and image similarity at R as high as 500. Figure 4 shows an example of the reconstructed HQ Mp 4D-MRI. Although the image quality was greatly degraded due to downsampling (Figure 4b), the reconstructed high-quality 4D-MR images still show detailed structures (Figure 4c, 4d) and accurate motion trajectory (Figure 4e). The average lesion motion errors are 1.37 mm and 1.23 mm for T1w and T2w HQ 4D-MRI among all the frames, respectively. D3R model inference takes about 60 ms, making the whole process within 500 ms.DISCUSSION & CONCLUSION
We successfully obtained HQ Mp 4D-MRI in real-time via the motion information estimated from ultra-downsampled radial k-space using the D3R model. The predicted DVFs from the D3R model are consistent for the down-sampling factor up to 500, and the lesion motion trajectory was accurately reflected in the 4D-MRI. Currently, 4D-MRI is rarely used in tumor tracking for its long acquisition time and poor quality. This technique shortened the total imaging time to 500 ms and enhanced its quality, making it more practical in RT.Acknowledgements
No acknowledgement found.References
1. Hewson EA, Dipuglia A, Kipritidis J, et al. First experimental evaluation of multi-target multileaf collimator tracking during volumetric modulated arc therapy for locally advanced prostate cancer. Radiother Oncol. 2021/07/01/ 2021;160:212-220. doi:https://doi.org/10.1016/j.radonc.2021.05.001
2. Wang C, Yin F-F. 4D-MRI in radiotherapy. IntechOpen; 2019.
3. Stemkens B, Paulson ES, Tijssen RH. Nuts and bolts of 4D-MRI for radiotherapy. Phys Med Biol. 2018;63(21):21TR01. doi:10.1088/1361-6560/aae56d
4. Keall PJ, Sawant A, Berbeco RI, et al. AAPM Task Group 264: The safe clinical implementation of MLC tracking in radiotherapy. https://doi.org/10.1002/mp.14625. Med Phys. 2021/05/01 2021;48(5):e44-e64. doi:https://doi.org/10.1002/mp.14625
5. Block KT, Chandarana H, Fatterpekar G, et al. Improving the robustness of clinical T1-weighted MRI using radial VIBE. Magnetom Flash. 2013;5:6-11.
6. Yang J, Cai J, Wang H, et al. Four-dimensional magnetic resonance imaging using axial body area as respiratory surrogate: initial patient results. Int J Radiat Oncol Biol Phys. 2014;88(4):907-912. doi:https://doi.org/10.1016/j.ijrobp.2013.11.245
7. Bedford JL, Fast MF, Nill S, et al. Effect of MLC tracking latency on conformal volumetric modulated arc therapy (VMAT) plans in 4D stereotactic lung treatment. Radiother Oncol. 2015/12/01/ 2015;117(3):491-495. doi:https://doi.org/10.1016/j.radonc.2015.07.044
8. Balakrishnan G, Zhao A, Sabuncu MR, Guttag J, Dalca AV. VoxelMorph: a learning framework for deformable medical image registration. IEEE Trans Med Imaging. Feb 4 2019;doi:10.1109/TMI.2019.2897538
Figures