4315
Improved Nuisance Signal removal for 1H-MRSI Using a Low-Rank Plus Sparse Model with Learned Subspaces
Xinyu Ye1,2, Zepeng Wang2,3, and Fan Lam2,3
1Center for Biomedical Imaging Research, Department of Biomedical Engineering, School of Medicine, Tsinghua University, Beijing, China, 2Department of Bioengineering, University of Illinois at Urbana-Champaign, Urbana, IL, United States, Urbana, IL, United States, 3Beckman Institute for Advanced Science and Technology, University of Illinois at Urbana-Champaign, Urbana, IL, United States
1Center for Biomedical Imaging Research, Department of Biomedical Engineering, School of Medicine, Tsinghua University, Beijing, China, 2Department of Bioengineering, University of Illinois at Urbana-Champaign, Urbana, IL, United States, Urbana, IL, United States, 3Beckman Institute for Advanced Science and Technology, University of Illinois at Urbana-Champaign, Urbana, IL, United States
Synopsis
Removing nuisance signals is an essential step for MRSI. A union-of-subspaces model that uses spatiospectral priors has achieved excellent water/lipid removal performance for 1H-MRSI, but may not be sufficient when the initial water/lipids are too strong and/or when field inhomogeneity is severe. We propose a low-rank plus sparse method for improved nuisance removal. The sparsity term is used to capture residual nuisance failed to be captured by the union-of-subspace model and the low-rank term with learned subspaces protects metabolite signals. Results from in vivo 1H-MRSI data show that the proposed method led to improved nuisance signal removal.
Introduction
Proton MRSI (1H-MRSI) provides unique spatially resolved molecular information for various physiological and pathological studies 1-2. A long-standing challenge for practical applications of 1H-MRSI is the strong water/lipids signals. These nuisance signals, sometimes overwhelming, prevent meaningful analysis of the metabolite signals if not effectively suppressed or removed. Both acquisition and postprocessing methods have been proposed to address this challenge 3-7. The union-of-subspaces (UoSS) model has demonstrated strong capability in nuisance signal removal (NSRM) from 1H-MRSI data by integrating spatial and spectral constraints on water/lipids 8-9. Nevertheless, substantial residual nuisance may still be present after UoSS NSRM, especially when the initial water/lipid signals are stronger (e.g., in short-TE and/or unsuppressed acquisitions) and/or in the regions with severe B0 inhomogeneity or imprecise spatial support information. We proposed here a strategy to further reduce these residual nuisance signals for improved MRSI data quality by synergizing sparsity constraints and data-adapted learned subspaces through the low-rank plus sparse modeling framework10.Theory
Low-Rank+Sparse (L+S) model for NSRMThe choice of L+S model was motivated by repeated observations that residual nuisance signals after UoSS processing typically appear as spatially sparse features (e.g., locally bright regions) with distinct residual water/lipid peaks and the low-rank property of the metabolite component 8-9. However, a direct application of the L+S model does not offer effective signal separation separation as (1) the low-rank component will compete with the sparse components in capturing nuisance components and (2) the sparse component may absorb metabolite signals leading to over removal. Therefore, we propose the following explicit low-rank+sparse formulation to address these issues:
$$C_{r,meta},S_{nu}=argmin\parallel d_{t} - \Omega F B(DC_{r,meta}\phi_{t}+S_{nu})\parallel_{2}^{2}+\alpha\parallel W(F_{t}(S_{nu})) \parallel_{1} (1)$$
where $$$C_{r,meta}\phi_{t}$$$ and $$$S_{nu}$$$ denotes the metabolite and nuisance components, respectively, with $$$C_{r,meta}$$$ and $$$\phi_{t}$$$ being low-rank matrices for the spatial coefficients and temporal basis, $$$d_{t}$$$ is (k,t)-space data, $$$\Omega$$$ an encoding operator, $$$F$$$ refers to 3D Fouerier transform, $$$B$$$ models B0 inhomogeneity induced phases, and $$$ D$$$ is a brain mask enforcing a spatial prior for metabolites.$$$W$$$ represents db4 wavelet tranform and $$$F_{t}$$$ refers to Fourier transform along t-dimension . The combination of $$$D$$$ and $$$\phi_{t}$$$ serves to protect the metabolite from being absorbed into $$$S_{nu}$$$ and also make $$$S_{nu}$$$ target the nuisance more specifically. The resulting optimization is solved using a fast iterative soft thresholding method12.
Estimation of the metabolite basis
Accurate estimation of metabolite basis $$$\phi_{t}$$$ plays an important role in our L+S model. One way to get the basis is applying frequency selective HSVD to the data itself or high-SNR calibration data, which is sensitive to noise and lineshape distortion. We propose to use a learned subspace approach11 (Fig.1). Specifically, we prelearned a metabolite subspace from previous training data then project residual signals onto the learned subspace. A parametric fitting with lineshape adaptation was then applied to the projection data to extract metabolite components and filter out residual nuisance projection. The fitted data was used to regenerate an adapted basis $$$\phi_{t}$$$.
Results
In vivo data were collected using a fast MRSI sequence13 on a Prisma 3T scanner (IRB approved) to evaluate the performance of our L+S method in further reducing the residual nuisance from UoSS NSRM. Fig.2 shows the NSRM results for one dataset produced w/wo the proposed L+S method. Compared to the original UOSS method, the L+S method further separated the residual nuisance signals into the sparse component (not shown here) and produced lower residuals. The L+S model with adapted basis further improved the removal performance. Fig.3 shows some representative spectra for different methods. Our L+S method further suppressed the residual water peaks at the voxels where UoSS did not perform well (voxels A&B) and produced similar spectra where UoSS performed well (voxel C), demonstrating the metabolite signal protection capability.The NSRM results for another volunteer are shown in Fig.4. The L+S method with either options of metabolite basis could further suppress the residual nuisance inside the brain and outside the brain. Note that reduction of strong residual nuisance outside the brain is still helpful as it can affect advanced processing such as low-rank filtering steps. Fig.5 shows reconstructed metabolite maps and localized spectra from data produced with only UoSS NSRM and UoSS plus our L+S NSRM. Improved spatiospectral data quality is apparent with improved NSRM offered by the proposed method.
Conclusion
We propose a low-rank plus sparse model to improve nuisance signal removal for 1H-MRSI data. A learned metabolite subspace adapted to the subject-specific experimental data is included to facilitate the separation of the sparse nuisance and low-rank metabolite components. We demonstrated that the proposed method can further remove nuisance signals after a state-of-the-art UoSS removal while protecting the metabolite signals, which lead to better metabolite spatiospectral reconstruction. We expect the proposed method to make 1H-MRSI more robust.Acknowledgements
No acknowledgement found.References
1.DeGraaf RA. In Vivo NMR Spectroscopy: Principles and Techniques, 3rd Edition. In Vivo Nmr Spectroscopy: Principles and Techniques, 3rd Edition 2019:1-560. 2.Maudsley AA, Domenig C, Govind V, et al. Mapping of Brain Metabolite Distributions by Volumetric Proton MR Spectroscopic Imaging (MRSI). Magn Reson Med 2009;61(3):548-559.3.Haase A, Frahm J, Hanicke W, Matthaei D. H1-Nmr Chemical-Shift Selective (Chess) Imaging. Phys Med Biol 1985;30(4):341-344.
4.Barkhuijsen H, Debeer R, Vanormondt D. Improved Algorithm for Noniterative Time-Domain Model-Fitting to Exponentially Damped Magnetic-Resonance Signals. J Magn Reson 1987;73(3):553-557.
5.Dong ZC, Hwang JH. Lipid signal extraction by SLIM: Application to H1-MR spectroscopic imaging of human calf muscles. Magnet Reson Med 2006;55(6):1447-1453.
6.Hernando D, Haldar J, Sutton B, Liang ZP. Removal of lipid signal in MRSI using spatial-spectral constraints. 2007 IEEE ISBI, Vols 1-3 2007:1360-+.
7.Bilgic B, Gagoski B, Kok T, Adalsteinsson E. Lipid suppression in CSI with spatial priors and highly undersampled peripheral k-space. Magn Reson Med 2013;69:1501-1511.
8.Ma C, Lam F, Johnson CL, Liang ZP. Removal of Nuisance Signals from Limited and Sparse H1-MRSI Data Using a Union-of-Subspaces Model. Magn Reson Med 2016;75(2):488-497.
9.Lam F, Ma C, Clifford B, Johnson CL, Liang ZP. High-Resolution H1-MRSI of the Brain Using SPICE: Data Acquisition and Image Reconstruction. Magn Reson Med 2016;76(4):1059-1070.
10. Otazo R, Candes E, Sodickson DK. Low-Rank Plus Sparse Matrix Decomposition for Accelerated Dynamic MRI with Separation of Background and Dynamic Components. Magnet Reson Med 2015;73(3):1125-1136.
11.Lam F, Li YD, Guo R, Clifford B, Liang ZP. Ultrafast magnetic resonance spectroscopic imaging using SPICE with learned subspaces. Magn Reson Med 2020;83:377-390.
12. Beck A, Teboulle M. A Fast Iterative Shrinkage-Thresholding Algorithm for Linear Inverse Problems. Siam J Imaging Sci 2009;2(1):183-202.
13.Wang Z, Li Y, Lam F. High-resolution, 3D multi-TE 1H-MRSI using fast spatiospectral encoding and subspace imaging, Magn Reson Med. In Press, 2021. DOI:10.1002/mrm.29015
Figures
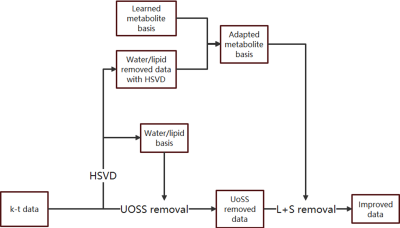
FIG. 1. Flowchart of the
proposed nuisance removal strategy with the L+S model-based removal.
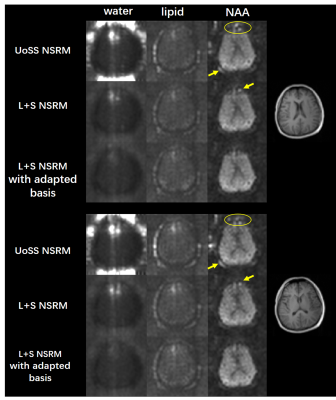
FIG. 2. Results from an vivo dataset with parameters: TR/TE=1200/30ms FOV=220×220×64mm3 matrix size=32(kx)×32(ky)×8(kz). Spectral integration of the residual water (col. 1), lipids (col. 2) and NAA peak (col. 3) after each removal step are shown for two representative slices. The proposed L+S method clearly further reduce the residual nuisance, especially when using the adapted subspace. Reduction of artifacts can be seen as highlighted by the yellow circles and arrows.
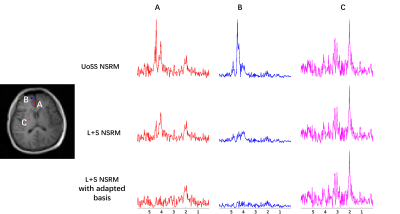
FIG. 3. Spectra from three
selected spatial points are compared for different methods. Three
rows show the results after UoSS removal, L+S removal and the L+S removal
with adapted subspace respectively. At voxel A and B, strong nuisance peaks
exist after the UoSS removal, while our proposed
L+S
method further suppress the residual water peaks especially with
adapted basis. At voxel C, UoSS performed well and L+S method produced similar spectra
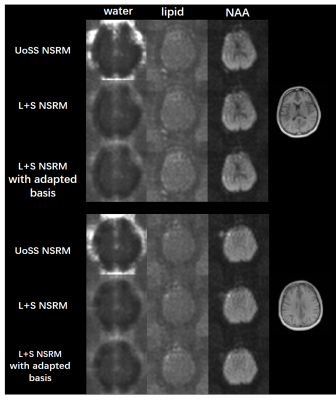
FIG. 4. Results from another dataset with the same acquisition parameter for two representative
slices. Spectral integration of the residual water
(col. 1), lipids (col. 2) and NAA peak (col. 3) after each removal step are
shown.
Suppression of residual nuisance can be observed in the
results from the L+S method with either option of metabolite basis.
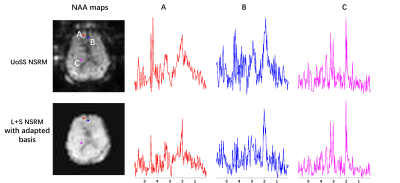
FIG. 5. Reconstructed metabolite maps and localized spectra from data produced
with UoSS NSRM and UoSS plus our L+S NSRM respectively. Compared to the results
from UoSS NSRM, the proposed method could improve the spatiospectral data
quality
DOI: https://doi.org/10.58530/2022/4315