4312
Accelerated VCC-Wave MR Imaging Using Deep Generative Models
Congcong Liu1,2, Zhuoxu Cui1, Zhilang Qiu1, Haoxiang Li1,2, Yifan Guo1, Chentao Cao1,2, Xin Liu1,2, Hairong Zheng1,2, Dong Liang1,2,3, and Haifeng Wang1,2
1Shenzhen Institutes of Advanced Technology,Chinese Academy of Sciences, Shenzhen, China, 2Shenzhen College of Advanced Technology, University of Chinese Academy of Sciences, Shenzhen, China, 3Research Centre for Medical AI, Shenzhen Institute of Advanced Technology, Chinese Academy of Sciences, Shenzhen, China
1Shenzhen Institutes of Advanced Technology,Chinese Academy of Sciences, Shenzhen, China, 2Shenzhen College of Advanced Technology, University of Chinese Academy of Sciences, Shenzhen, China, 3Research Centre for Medical AI, Shenzhen Institute of Advanced Technology, Chinese Academy of Sciences, Shenzhen, China
Synopsis
CSM or kernel needs to be estimated in conventional parallel image, which is very time-consuming and the estimation process may be inaccurate. Here, wave coding model based on virtual conjugate coil using deep generative modes (WV-DGM) is proposed for the virtual conjugate coil (VCC) extended model based on wave coding. WV-DGM combined with deep DGM without training can realize advantages of wave encoding and introduce extra phase to reduce the ill-condition of the model via VCC. The results in vivo demonstrated that WV-DGM can achieve better quality compared with conventional SENSE while 10x times used to estimate CSM is reduced.
Introduction
In MRI reconstruction based on wave encoding, the condition number of the system can be reduced by using the re-encoded coil sensitivity information of the readout direction. Coil sensitivity maps (CSM) need to be estimated prior common reconstruction using SENSE[1-4] and kernels need to be calculated in the GRAPPA[5-7] for calibration in k-space. The process of calculation the CSM or kernel is laborious and inaccurate while having requirement to the template. A large amount of training data needs to be collected and preprocessed in using conventional deep learning reconstruction methos[8],[9], which is difficult to achieve in clinical[10]. Fortunately, deep generative models[11],[12] (DGM) are a kind of untrained net including CSM free and calibration free. In addition, DGM maintains the equal reconstruction accuracy while still maintaining a theory similar to CS to ensure that the physical encoding process meets certain conditions[13],[14].A new model based on wave coding model[15] and combined with virtual conjugate coil (VCC) without untraning was proposed in this work, named WV-DGM. WV-DGM not only contains wave encoding mechanism, but also has the efficiency and accuracy of DGM. Furthermore, additional phase information is also considered to improve the reconstruction. WV-DGM has achieves the best reconstruction quality in vivo data while having the shortest reconstruction time compared to other reconstruction methods. We believe that WV-DGM will have greater potential in clinical application to achieve rapid reconstruction.
Methods
The DGM (as shown in Fig. 1) employed in the WV-DGM is that presents a generative convolutional neural network that provides a low-dimensional to high-dimensional mapping method [8], i.e., $$$G: \mathbb{R}^p \to \mathbb{R}^{c \times w \times h }$$$, where $$$c$$$, $$$w$$$, and $$$h$$$ represent the number of output channel, the width and height of the image each channel, respectively. The input of DGM is fixed, chosen randomly. The VCC-based wave coding (Wave-VCC) can effectively utilize the physical properties in MRI and increase the SNR of the reconstruction. WV-DGM is proposed combined with DGM as follows, $$arg\min_{ \mathcal {G}_i} \frac {1} {2} \sum_{i=1}^{\mathcal {n}_\mathcal {c}} || \mathcal {M} \mathcal {F}_x^{-1} \mathcal {Psf}( \mathcal{k}_\mathcal{i}^{\ast}, \mathcal{y}) \mathcal {F}_\mathcal {x} \mathcal {G}_\mathcal{i}(\xi)- \mathcal {k}_\mathcal{i}^{\ast} ||_2^2$$.Where, a set of $$$\mathcal {k}_\mathcal{i}^{\ast}$$$ k-space data consisted of $$$\mathcal {k}_1^{\ast}$$$, $$$\cdots$$$, $$$\mathcal {k}_\mathcal {n_c}^{\ast}$$$ of $$$\mathcal {n}_\mathcal{c}$$$ receiver coil is given with a given mask $$$\mathcal {M}$$$ of an unknown image, especially, $$$\mathcal{k}_\mathcal{i}^{\ast}$$$ were expanded from the original $$$\mathcal {k}_\mathcal {i}$$$ data via VCC. $$$\mathcal {Psf}(\mathcal {k}_\mathcal {i}^{\ast},\mathcal{y})$$$ was introduced as a kind of convolutional kernel in the readout direction in MRI, which can effectively reduce the ill-condition of WV-DGM solution and increase SNR.
The IRB (institutional review board) approved in vivo human brain experiments were performed on a 3T uMR790 scanner (United Imaging, Shanghai, China). The WV-DGM is introduced into the common PI based on Wave-VCC physical encoding, which can greatly reduce the reconstruction complexity and time due to the steps of CSM being estimated in SENSE, or kernel in GRAPPA are eliminated. The knee data was encoded and expanded by using the process of simulated via wave coding and the expend of VCC methods. The results show that WV-DGM achieves better results compared with conventional SENSE reconstruction. The brain data of the gradient field encoded by the actual wave was also collected and expanded with the VCC. The results demonstrate that WV-DGM proposed in this work has better performance compared to SENSE. In addition, out method is more friendly to clinical realization.
Results
In Fig. 2, the Psf divided into original and extended via VCC was measured in the actual scanning system. The 7-cycles wave were successfully implemented for the actual encoding system, which can reduce the ill-condition in WV-DGM. The simulated Psf was generated to encode the knee data (4x) to reconstruction, as shown in Fig. 3. The results show that the reconstruction quality by using the DMG model has gradually improved in V-DGM, W-DGM, and DGM, especially, WV-DGM can reconstruct the best image quality including higher SNR and contrast while 10x times used to estimate CSM is reduced. The brain data in vivo encoded by wave was reconstructed in scanner to verify the actual reconstruction quality of WV-DGM. WV-DGM shows that our proposed WV-DGM technique enhances the reconstruction, as shown in Fig. 4.Conclusion
In summary, WV-DGM greatly reduces the complexity of reconstruction without needing to estimate CSM or kernel. This technology is friendly in clinical implementation.Acknowledgements
Congcong Liu and Zhuoxu Cui contributed equally to this work. The authors thank Prof. Berkin Bilgic and Mohammad Zalbagi Darestani for sharing the WaveCAIPI, and ConvDecoder reconstruction codes. Some of the work was partially supported by the National Natural Science Foundation of China (61871373, 81729003, and 81901736), the Strategic Priority Research Program of Chinese Academy of Sciences (No. XDB25000000), the Chinese Academy of Sciences Engineering Laboratory for Medical Imaging Technology and Equipment (No. KFJ-PTXM-012), the Pearl River Talent Recruitment Program of Guangdong Province (2019QN01Y986), the Shenzhen Science and Technology Program (JCYJ20210324115810030), the Shenzhen Peacock Plan Team Program (No. KQTD20180413181834876), and the Shenzhen Key Laboratory of Ultrasound Imaging and Therapy (No. ZDSYS201802061806314).References
- Pruessmann, Klaas P., et al. "SENSE: sensitivity encoding for fast MRI." Magnetic Resonance in Medicine: An Official Journal of the International Society for Magnetic Resonance in Medicine 42.5 (1999): 952-962.
- Weiger, Markus, Klaas P. Pruessmann, and Peter Boesiger. "2D SENSE for faster 3D MRI." Magnetic Resonance Materials in Physics, Biology and Medicine 14.1 (2002): 10-19.
- Wang, Haifeng, et al. "Parameter optimization framework on wave gradients of Wave‐CAIPI imaging." Magnetic resonance in medicine 83.5 (2020): 1659-1672.
- Su, Shi, et al. "Accelerated 3D bSSFP using a modified wave-CAIPI technique with truncated wave gradients." IEEE Transactions on Medical Imaging 40.1 (2020): 48-58.
- Griswold, Mark A., et al. "Generalized autocalibrating partially parallel acquisitions (GRAPPA)." Magnetic Resonance in Medicine: An Official Journal of the International Society for Magnetic Resonance in Medicine 47.6 (2002): 1202-1210.
- Blaimer, Martin, et al. "2D‐GRAPPA‐operator for faster 3D parallel MRI." Magnetic Resonance in Medicine: An Official Journal of the International Society for Magnetic Resonance in Medicine 56.6 (2006): 1359-1364.
- Qiu, Zhilang, et al. "Highly accelerated parallel MRI using wave encoding and virtual conjugate coils". Magnetic resonance in medicine 86.3 (2021): 1345-1359.
- Han, Yoseo, Leonard Sunwoo, and Jong Chul Ye. "k-Space Deep Learning for Accelerated MRI." IEEE transactions on medical imaging 39.2 (2019): 377-386.
- Bahadir, Cagla D., et al. "Deep-learning-based optimization of the under-sampling pattern in MRI." IEEE Transactions on Computational Imaging 6 (2020): 1139-1152.
- Chun, Jaehee, et al. "MRI super‐resolution reconstruction for MRI‐guided adaptive radiotherapy using cascaded deep learning: In the presence of limited training data and unknown translation model." Medical physics 46.9 (2019): 4148-4164.
- Darestani, Mohammad Zalbagi, and Reinhard Heckel. "Accelerated MRI with un-trained neural networks." IEEE Transactions on Computational Imaging 7 (2021): 724-733.
- Arora, S., V. Roeloffs, and M. Lustig. "Untrained modified deep decoder for joint denoising parallel imaging reconstruction." International Society for Magnetic Resonance in Medicine Annual Meeting. 2020.
- Haldar, Justin P., Diego Hernando, and Zhi-Pei Liang. "Compressed-sensing MRI with random encoding." IEEE transactions on Medical Imaging 30.4 (2010): 893-903.
- Quan, Tran Minh, Thanh Nguyen-Duc, and Won-Ki Jeong. "Compressed sensing MRI reconstruction using a generative adversarial network with a cyclic loss." IEEE transactions on medical imaging 37.6 (2018): 1488-1497.
- Bilgic, Berkin, et al. "Wave‐CAIPI for highly accelerated 3D imaging." Magnetic resonance in medicine 73.6 (2015): 2152-2162.
Figures
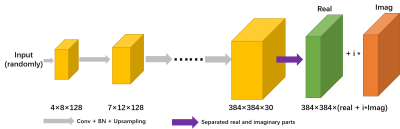
Fig. 1 DGM architecture based on Wave and VCC. It is comprised of up-sampling, convolutional, ReLU, batch normalization, and linear combination layer.
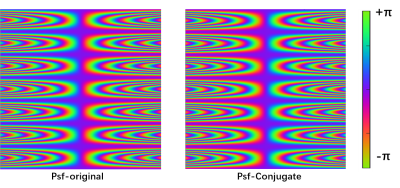
Fig. 2 The real acquisition Psf
expanded by VCC is divided into original part and conjugate part. The voxel in
readout direction is expanded due to the introduction of Psf, which can reduce
the ill-condition in WV-DGM.
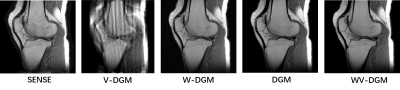
Fig. 3 The knee data is reconstructed
through the simulated Psf. In the above results of reconstruction based on DGM,
i.e., V-DGM (VCC-DGM), W-DGM (Wave-DGM), WV-DGM can achieve the best
reconstructed SNR and contrast compared to common SENSE. All data is
reconstructed at a 4 times acceleration (4x).
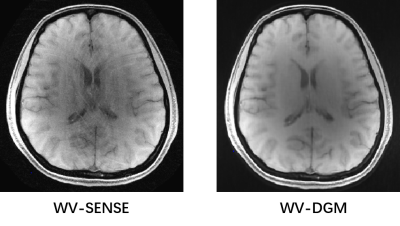
Fig. 4 Comparison result of reconstruction of WV-DGM and WV-SENSE (Wave-VCC
SENSE) over brain data in vivo. The results illustrated that WV-DGM can reconstrued
better quality (4x).
DOI: https://doi.org/10.58530/2022/4312