4292
Gadolinium-free Contrast-enhanced MRI (GFCE-MRI) Synthesis via Generalizable MHDgN-Net for Patients with Nasopharyngeal Carcinoma1The Hong Kong Polytechnic University, HONG KONG, Hong Kong, 2Queen Elizabeth Hospital, HONG KONG, Hong Kong, 3The University of Hong Kong, HONG KONG, Hong Kong, 4Hong Kong Sanatorium & Hospital, HONG KONG, Hong Kong
Synopsis
We have developed and validated a MHDgN-Net for gadolinium-free contrast-enhanced MRI (GFCE-MRI) synthesis in patients with nasopharyngeal carcinoma (NPC). The developed MHDgN-Net was featured with high generalizability. We first modelled the MHDgN-Net using three hospital datasets to improve the diversity of training samples. Then, the external hospital data was matched to the distribution of training dataset by EDM. Compared to traditional models, the proposed MHDgN-Net can accurately enhance tumor and significantly improve the quality of GFCE-MRI when applying to external hospital data. This technique holds great potential in providing a generalizable gadolinium-free tumor enhancement alternative on data from other hospitals.
Introduction
Nasopharyngeal carcinoma (NPC) is a highly aggressive malignancy that has long been observed in the population of East and Southeast Asia1. Radiotherapy (RT) is currently the mainstay treatment modality. In a successful RT treatment, precision tumor delineation is the most critical prerequisite. Contrast-enhanced MRI (CE-MRI) through injection of gadolinium-based contrast agents (GBCAs) plays a key role in tumor delineation owing to its excellent tumor-to-normal tissue contrast. Nonetheless, accumulated evidence has indicated that gadolinium exposure has been strongly associated with allergic reactions and fatal nephrogenic systemic fibrosis2. Recently, deep learning-assisted gadolinium-free CE-MRI (GFCE-MRI) has been developed as an alternative to reduce or eliminate the use of GBCAs3-5. However, existing GFCE-MRI models suffer from a bench-to-bedside deficiency in low or unknown model generalizability4,5. To address this issue, a generalizable multi-hospital data-guided neural network (MHDgN-Net) was proposed and evaluated in this study for precision tumor delineation in patients with NPC.Methods
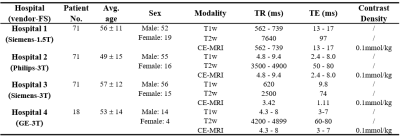
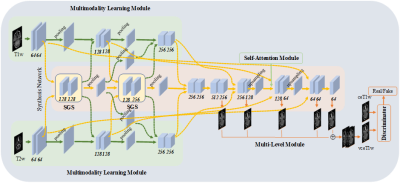
Data description: A total of 231 patients were enrolled from Queen Elizabeth Hospital and three affiliated hospitals of The University of Hong Kong (labeled as hospital 1, …, hospital 4). The number of enrolled patients in each hospital was 71, 71, 71, 18 respectively. From hospital 1 to hospital 3, each dataset was randomly split into 53 and 18 patients for model training and internal evaluation. The 18 patients from hospital 4 were used for external evaluation. All enrolled patients were scanned with contrast-free T1-weighted (T1w) and T2-weighted (T2w) MRI, and GBCA-based CE-MRI. The details of patient characteristics were illustrated in Table 1. The T1w and T2w MRI were utilized as input, and CE-MRI was used as the learning target.Study design: The proposed MHDgN-Net is a two-stage approach, including mixture modeling (MM) and external distribution matching (EDM). The MHDgN-Net was featured with high generalizability, which largely relies on the diversity of training samples. At the stage of MM, we aimed at increasing the diversity of training samples. We first integrated training data from hospital 1 to hospital 3 to construct a mixture dataset. To keep the patient number of mixture dataset consistent with single hospital datasets, 18 patients were randomly sampled from each hospital. Then one patient was randomly excluded, resulting in 53 patient samples in the mixture dataset. Next, the mixture dataset was trained to generate a mixture model. The network architecture was built based on our previously developed MMgSN-Net6, as shown in Figure 1. For comparison, three benchmark models were trained separately using the dataset from each single hospital (53 patients). Different hospital data was scanned with various imaging protocols and scanners, resulting in various intensity distributions, which greatly reduces the generalizability of deep learning models. At the stage of EDM, we aimed at improving the model generalizability indirectly by minimizing the intensity variation of external data. The external dataset was matched with the mixture dataset by y=(μ1/μ2)*x, where x means the pixel value of external dataset, μ1and μ2 are slice-based mean value of the overall mixture dataset and external dataset, respectively. y is the matched pixel value. The external dataset after distribution matching (hospital 4_M) got the same mean pixel value as the training dataset, therefore the data distribution variation of the external dataset could be minimized. Different from the widely used z-score normalization, EDM can reserve the scale of the original data. Lastly, the hospital 4_M was input to the mixture model for GFCE-MRI synthesis.
Results
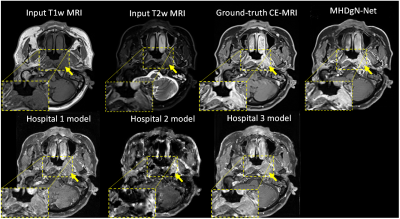
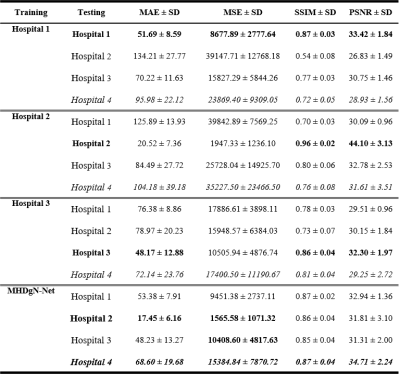
Visual comparison between the generated GFCE-MRI from single hospital models and MHDgN-Net was shown in Figure 2. The image quality of GFCE-MRI generated from MHDgN-Net was largely improved, especially in the tumor region. Mean Absolute Error (MAE), Mean Squared Error (MSE), Structural Similarity Index (SSIM), and Peak Signal-to-Noise Ratio (PSNR) between GBCA-based CE-MRI and generated GFCE-MRI were calculated for quantitative comparison. Detailed quantitative results were listed in Table 2. For internal evaluation, single hospital models obtained the best results on training hospital data. Against single hospital models, MHDgN-Net achieved comparable internal quantitative results on all internal hospital datasets. Additionally, in the external evaluation, MHDgN-Net also achieved the best result on hospital 4 dataset with the lowest MAE, MSE of 68.60 ± 19.68 and 15384.84 ± 7870.72, and the highest SSIM and PSNR of 0.87 ± 0.04 and 34.71 ± 2.24 respectively.Discussion
In this study, we developed a generalizable MHDgN-Net for GFCE-MRI synthesis in patients with NPC. The quantitative results showed that single hospital models can perform well on training hospital data, but with poor generalizability to other hospital images. The proposed MHDgN-Net that trained with the same number of training samples outperformed single hospital models in both internal and external evaluations, showing that MHDgN-Net has higher generalizability against single hospital models. The quantitative results of the hospital 2 model were significantly better than the other two single hospital models, which is expected since this hospital data have smaller intensity value.Conclusion
A generalizable MHDgN-Net was developed for eliminating the use of GBCAs in NPC patients. The synthetic GFCE-MRI showed accurate tumor enhancement and significantly improved image quality on both internal and external hospital datasets. The developed technique has high potential to provide an alternative for precision tumor delineation without administration of GBCAs.Acknowledgements
This research was partly supported by funding GRF 151022/19M and ITS/080/19.References
1. Chang ET, Ye W, Zeng YX, Adami HO. The evolving epidemiology of nasopharyngeal carcinoma. Cancer Epidemiology and Prevention Biomarkers. 2021; 30(6): 1035-47.
2. Gong E, Pauly JM, Wintermark M, Zaharchuk G. Deep learning enables reduced gadolinium dose for contrast‐enhanced brain MRI. Journal of magnetic resonance imaging. 2018; 48(2):330-40.
3. Kleesiek J, Morshuis JN, Isensee F, Deike-Hofmann K, Paech D, Kickingereder P, Köthe U, Rother C, Forsting M, Wick W, Bendszus M. Can virtual contrast enhancement in brain MRI replace gadolinium? a feasibility study. Investigative radiology. 2019; 54(10): 653-60.
4. Luo H, Zhang T, Gong NJ, Tamir J, Venkata SP, Xu C, Duan Y, Zhou T, Zhou F, Zaharchuk G, Xue J. Deep learning–based methods may minimize GBCA dosage in brain MRI. European Radiology. 2021; 31: 6419-28.
5. Chen C, Raymond C, Speier B, Jin X, Cloughesy TF, Enzmann D, Ellingson BM, Arnold CW. Synthesizing MR Image Contrast Enhancement Using 3D High-resolution ConvNets. arXiv preprint arXiv:2104.01592. 2021 Apr 4.
6. Li W, Xiao H, Li T, Ren G, Lam S, Teng X, Liu C, Zhang J, Lee FK, Au KH, Lee VH, Chang ATY, Cai J. Virtual Contrast-enhanced Magnetic Resonance Images Synthesis for Patients with Nasopharyngeal Carcinoma using Multimodality-guided Synergistic Neural Network. International Journal of Radiation Oncology* Biology* Physics. Accepted in 2021.
Figures