4288
Golden-angle radial sparse parallel (GRASP) and simultaneous multi-slice RESOLVE DWI MRI in evaluation of postoperative pituitary adenomas1Neurosurgery, the First Hospital of Jilin University, Changchun, China, 2Otorhinolaryngology Head and Neck Surgery, the First Hospital of Jilin University, Changchun, China, 3Radiology, the First Hospital of Jilin University, Changchun, China, 4Siemens Healthineers ., Beijing, China, 5Siemens Healthineers Digital Technology., Harbin, China
Synopsis
MRI is a primary imaging method for the evaluation of pituitary adenomas (PAs). However, it is challenging to identify the postoperative residual tumors using traditional techniques. A recent approach Golden-angle Radial Sparse Parallel (GRASP)-DCE, and the novel simultaneous multi-slice (SMS) readout-segmented EPI technique (RESOLVE) procedures were performed to assess the residual tumors of postoperative PAs. And the residual tumors were observed as slow enhancement in GRASP images while manifesting as hyperintense in SMS-RESOLVE images. Thus, both of them are promising techniques for the evaluation of postoperative residual PAs.
Introduction
Magnetic resonance imaging (MRI) is a primary imaging method for evaluating pituitary adenomas (PAs). T1W, T2W, and dynamic contrast-enhanced (DCE) MRI are routinely used for preoperative and postoperative evaluation in clinical examinations. However, it is challenging to identify the postoperative residual tumors using traditional techniques.A recent approach, Golden-angle Radial Sparse Parallel (GRASP)-DCE MRI, combining a continuously acquired radial k-space trajectory with golden-angle sampling and compressed sensing-based sparse parallel reconstruction, can offer DCE data with simultaneously high spatial and temporal resolution1. Furthermore, it can increase sensitivity through higher temporal and spatial resolutions in depicting pituitary microlesions2,and is a promising method for evaluating postoperative residual PAs. Diffusion-weighted imaging (DWI) is a widely used technique for evaluating tumors without contrast agents and could be a complement to assess the PAs. However, traditional single-shot echo-planar imaging (SS-EPI) DWI suffers from severe susceptibility artifacts and distortion near the skull base and thus has limited applicability in pituitary imaging, especially in microlesions3. The readout-segmented EPI technique (RESOLVE) was recently applied in clinical routine and can significantly reduce distortions and artifacts by reducing echo spacing and TE. However, long scanning time is a limitation, especially for high-resolution DWI4. A novel simultaneous multi-slice (SMS) technique may accelerate acquisitions by exciting and acquiring signals from multiple slices simultaneously, thus significantly reducing the scan time of RESOLVE5.The purpose of this study was to assess the diagnostic performance of GRASP-DCE and SMS-RESOLVE DWI MRI for identifying the residual tumors in the evaluation of the postoperative PAs.Methods
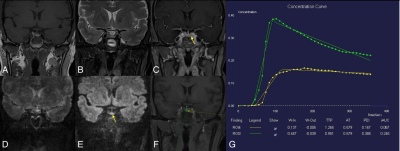
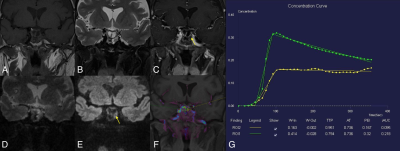
Three patients ( mean age: 50 years old, 3 female) of postoperative PAs and two healthy female volunteers were enrolled in this study. T1WI, T2WI, GRASP DCE, and SMS-RESOLVE DWI were performed on 3T MRI systems (MAGNETOM Vida, Siemens Healthcare, Erlangen, Germany). GRASP-DCE data were acquired using the following parameters: temporal resolution of 9.4 sec/phase for 31 periods TR/TE=3.96/1.98ms, FOV=205x205mm2, spatial resolution=0.8x0.8mm2, slice thickness=2.5mm, number of slices=20, total acquisition time=6min15se. For post-processing, the quantitative perfusion analysis was performed using a standard Tofts model (Tissue4D, Siemens Healthcare) to generate the volume transfer coefficient (Ktrans, min-1), rate constant between extracellular/extravascular space (EES) (Kep,min-1), and the interstitial volume (Ve, ml/ml). SMS-RESOLVE scanning parameters are as follows: TR/TE1/TE2=3000/82/50ms, FOV=250x125mm2, spatial resolution=0.7x0.7mm2, slice thickness=2mm, number of slices=28, readout segments =9, echo spacing=0.30ms, bandwidth = 766Hz/Px, total acquisition time=2min48se. Two radiologists independently interpreted the postoperative PAs MR images in random order on the local Siemens workstation. The images with the following features were assessed: with or without the postoperative residue of PAs on GRASP or SMS-RESOLVE DWI. Regions of interest (ROIs) were placed in GRASP images to measure the level of enhancement. One radiologist derived ROI in images of GRASP. In GRASP images, the uniform and prominent enhanced areas were considered normal pituitary tissue (green circle), while the delayed enhanced area was considered residual PAs (yellow circle). Based on the characteristic of slow enhancement of PAs6, the concentration curves of normal pituitary tissue and PAs can further verify the accuracy of the diagnosis.Results & Discussion
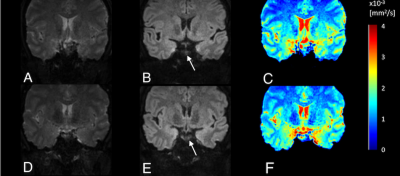
Three patients of postoperative PAs have identified the residual tumors on GRASP images and the SMS-RESOLVE DWI images with the agreement of the two radiologists. Figure 1 shows images of 2 healthy volunteers without PAs scanned by SMS-RESOLVE DWI. SMS-RESOLVE DWI could clearly show pituitaries. Figure 2 and figure 3 are images of 2 patients who underwent postoperative review after transsphenoidal pituitary adenoma resection. It can be observed that SMS-RESOLVE DWI preserves high structural consistency with GRASP. Residual postoperative PAs shown on GRASP can also be shown on SMS-RESOLVE DWI in the same places. Residual tumors show slow enhancement in GRASP images while manifesting as hyperintense in SMS-RESOLVE images. On GRASP images, the uniform and prominent enhanced areas were considered normal pituitary tissue (green circle), while the delayed enhanced area was considered PAs (yellow circle). Concentration curves proved that areas identified as lesions in images answer the description of slow enhancement of PAs. The enhancement of lesions was more minor and delayed compared with the surrounding normal pituitary tissue. Thus, the residual tumor was diagnosed in these patients.Conclusion
In the postoperative evaluation of pituitary adenomas, the novel GRASP DCE and SMS-RESOLVE DWI methods, which can obtain high-resolution and high-quality images, and the shorter scan time, may show the potential high diagnostic performance in the identification of the residual tumors. Future research needs acquisitions of more patients and quantitative analysis.Acknowledgements
No acknowledgement was found.References
1.Feng L, Grimm R, Block KT, Chandarana H, Kim S, Xu J, et al. Golden-angle radialsparse parallel MRI: combination of compressed sensing, parallel imaging, andgolden-angle radial sampling for fast and flexible dynamic volumetric MRI. MagnReson Med. 2014;72(3):707–717.
2. Nicolin H, Christoph S, Julia R, et al. Golden-angle radial sparse parallel (GRASP) MRI in clinical routine T detection of pituitary microadenomas: First experience and feasibility. Magnetic Resonance Imaging. 2019;60:38–43.
3. Rennert J, Doerfler A. Imaging of sellar and parasellar lesions. Clinical Neurology and Neurosurgery. 2007;109(2):111-124.
4. Lu, P., G. Tian, X. Liu, F. Wang, Z. Zhang and Y. Sha. Differentiating Neuromyelitis Optica-Related and Multiple Sclerosis-Related Acute Optic Neuritis Using Conventional Magnetic Resonance Imaging Combined with Readout-Segmented Echo-Planar Diffusion-Weighted Imaging. World J Surg Oncol. 2018;42(4):502-509.
5. Jiang, J. S., Zhu, L. N., Wu, Q., Sun, Y., Liu, W., Xu, X. Q., & Wu, F. Y. (2020). Feasibility study of using simultaneous multi-slice RESOLVE diffusion weighted imaging to assess parotid gland tumors: comparison with conventional RESOLVE diffusion weighted imaging. BMC medical imaging, 2020;20(1), 93.
6.Rossi Espagnet MC, Bangiyev L, Haber M, Block KT, Babb J, Ruggiero V, et al. High- resolution DCE-MRI of the pituitary gland using radial k-space acquisition with compressed sensing reconstruction. AJNR Am J Neuroradiol. 2015;36(8):1444–1449.
Figures