4280
Time-dependent IVIM and non-Gaussian parameters in breast cancer and melanoma xenograft models; correlation with histological markers1Department of Diagnostic Imaging and Nuclear Medicine, Kyoto University, Kyoto, Japan, 2Institute for advancement of clinical and translational science, Kyoto University Hospital, Kyoto, Japan, 3Department of Systems science, Graduate School of Informatics, Kyoto University, Kyoto, Japan, 4Department of Diagnostic Pathology, Kyoto University, Kyoto, Japan, 5NeuroSpin/Joliot, CEA-Saclay Center, Paris-Saclay University, Gif-sur-Yvette, France, 6Human Brain Research Center, Kyoto University Graduate School of Medicine, Kyoto, Japan, 7National Institute for Physiological Sciences, Okazaki, Japan
Synopsis
Time-dependent IVIM and non-Gaussian diffusion parameters might provide additional information over conventional DWI to evaluate features linked to tissue microstructure. Time-dependent IVIM and non-Gaussian diffusion parameters were evaluated both in-vivo and post-mortem in MDA-MB-231 (breast cancer) and B16 (melanoma) models. As expected, fIVIM values significantly dropped from in-vivo to post-mortem conditions with both models at two different diffusion times, confirming the association between fIVIM and tumor perfusion, but did not reach 0, suggesting limitations in the IVIM/non-Gaussian Kurtosis model. ADCo values significantly decreased at long diffusion times and upon sacrification while Kurtosis moderately increased.
Introduction
IVIM MRI has the potential to provide quantitative information on microcirculation (perfusion) non-invasively, and the increase of fIVIM (perfusion-related fraction) has been reported as associated with the proliferation of neovessels in some cancer tumors1. Thus, IVIM parameters are expected to play an important role as imaging biomarkers to reveal and monitor tumor angiogenesis without the need for a contrast agent. Evaluating the degree of tumor angiogenesis is important for diagnosis, characterization, or staging of malignant tumors. However, fitting data to the IVIM model sometimes fails, revealing some issues, such as effects of non-Gaussian diffusion and noise floor issues2. While a decrease in fIVIM values after sacrification is expected with the association between IVIM parameters and tumor perfusion, as reported previously3, fIVIM does not always reach 0, which has been ascribed to limitations to the IVIM/Kurtosis model.Here, we investigated IVIM and non-Gaussian diffusion parameters in-vivo and post-mortem conditions using two different tumor cell lines (breast cancer and melanoma) and evaluated the association between these parameters and pathological information, such as microvessel density and vessel size.
Materials & Methods
Seven ICR nu/nu mice and seven BALB mice were injected subcutaneously with 1×106 MDA-MB-231 cells and B16-F0-Lac cells, respectively in the hind limb. MRI was conducted on a 7T MRI scanner (Bruker, Germany) using a 1H quadrature transmit/receive volume coil. The SE-EPI acquisition parameters were set as follows; resolution 250x250μm², FOV 25x25 mm², slice-thickness 1.5 mm, TE=57ms, TR=2500ms, 8averages, 4segments. IVIM/Diffusion-weighted (DW) images were acquired using 2 different diffusion times (Td, 9 and 27.6ms) and 19 b values (7-4105 s/mm²). MRI images were obtained both in-vivo and post-mortem. The total acquisition time was 22min 40sec.Shifted ADC (sADC) values were calculated using b values of 200 and 1500 sec/mm2 4. Non-Gaussian diffusion and fIVIM values were estimated using the combined IVIM/non-Gaussian diffusion kurtosis model corrected for Noise Floor effects5. Xenograft tumors were harvested and stained immunohistochemically for CD31. CD31-positive microvessel density (MVD) and diameters of microvessels (minor axis) were measured using QuPath (version 0.3.0, open-source software). MRI data analysis was performed using a code developed in Matlab (Mathworks, Natick, MA). Diffusion parameters were compared using the paired-t test and Wilcoxon test, and the correlation of MR parameters with histopathological parameters were investigated using the Spearman's rank correlation test.
Results
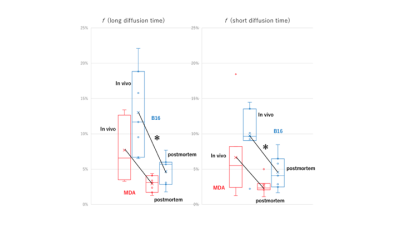
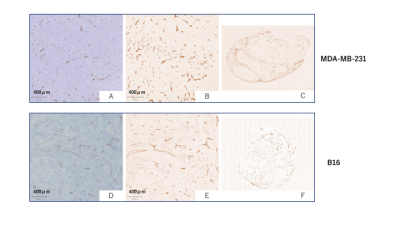
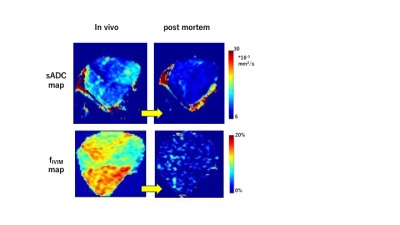
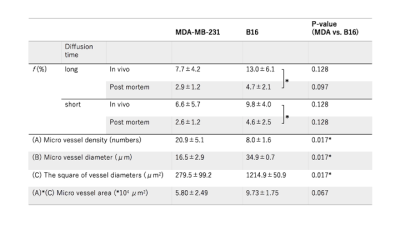
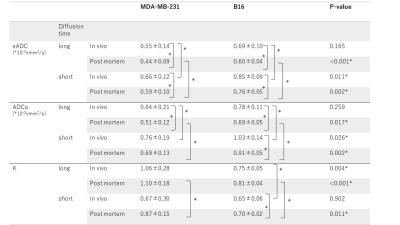
The fIVIM values significantly dropped after sacrification in B16 tumors (Mean±SD; 13.0±6.1% vs. 5.0±2.1%; P<0.05 at long-Td and 9.8±4.0% vs. 4.8±2.7%; P<0.05 at short-Td) and tended to decrease in post-mortem condition in MDA-MB-231 tumors (7.7±4.2% vs. 2.9±1.2%; P=0.058 at long-Td and 6.6±5.7% vs. 2.6±1.2%; P=0.124 at short-Td). However, fIVIM values after sacrification did not reach 0 despite the lack of perfusion, as previously reported3. fIVIM in B16 tended to be larger than MDA-MB-231 at both Td and in both in-vivo and post-mortem conditions (Fig. 1). CD31-positive MVD tended to be larger in MDA-MB-231 compared to B16 tumors (Table 1). In MDA-MB-231, numerous blood vessels with small round lumens were observed while B16 tumors had blood vessels with irregular, expanded lumens (Fig. 2). There was no significant positive association between fIVIM and MVD, but there was a correlation between fIVIM and the square of the vessel's diameters (vessel lumen area) (R=0.75, P=0.012). In both tumors, sADC values significantly decreased both when increasing the Td and after sacrification (Table 2, P<0.05, respectively). ADCo values had almost the same behavior as sADC, however, the short-Td ADCo decrease after sacrification, did not reach significance (P=0.06). K after sacrification showed a mild increase for both tumors and both Td.Discussion
The absence of correlation in-vivo of fIVIM with MVD, while they were correlated with the vessels lumen area (more vascularization and larger capillary lumen space for B16 compared with MDA-MB-231 model6), confirms that fIVIM corresponds more to blood volume than vessel density. A significant decrease of fIVIM values after sacrification was observed both in breast cancer and melanoma xenograft models, confirming the strong association between fIVIM and tumor microperfusion, but not reaching 0. Residual post-mortem fIVIM in B16 tended to be slightly higher (5%) than MDA-MB-231 (3%) independently of Td. Those residual fIVIM effects may result from imperfect fitting of the data with the IVIM/Kurtosis model which has known limitations. However, as ADCo (and sADC) values were also higher for the B16 model we may hypothesize that the larger capillary volume might represent a pool of fast diffusing (quasi-free) water molecules in tumor capillaries, larger for B16 than MDA. This pool would artifactually contribute to the IVIM compartment, as D* in the IVIM model must be taken as the sum of the perfusion-driven pseudo-diffusion coefficient and the water diffusion coefficient in the vessel which remains present even in the absence of blood flow. This hypothesis was supported by simulations using data from this study, however, further work is in progress to investigate this hypothesis.Conclusion
Our findings confirm that fIVIM values reflect blood microcirculation in tissues more in terms of volume than vessel density. Residual post-mortem fIVIM values may suggest the imperfections in the IVIM/Kurtosis model or the sensitivity of the model to water pools with high diffusion, close to the D* values expected by the perfusion-driven IVIM model, as, thus contaminating fIVIM values.Acknowledgements
The authors thank the Center for Anatomical, Pathological and Forensic Medical Research, Kyoto University Graduate School of Medicine, for preparing microscope slides.References
1. Iima, M. Perfusion-driven Intravoxel Incoherent Motion (IVIM) MRI in Oncology: Applications, Challenges, and Future Trends. Magn. Reson. Med. Sci. 20:125-138 (2021).
2. Iima, M. & Le Bihan, D. Clinical Intravoxel Incoherent Motion and Diffusion MR Imaging: Past, Present, and Future. Radiology 278, 13–32 (2016).
3. Yuko Someya, Mami Iima, Hirohiko Imai, Hiroyoshi Isoda, Masako Kataoka, Denis LeBihan, Yuji Nakamoto. Time-dependent IVIM/Non-Gaussian parameters between in vivo and post-mortem breast cancer xenograft models. ISMRM & SMRT Annual Meeting & Exhibition #1150 (2021).
4. Iima, M. et al. Effects of diffusion time on non-Gaussian diffusion and intravoxel incoherent motion (IVIM) MRI parameters in breast cancer and hepatocellular carcinoma xenograft models. Acta Radiol Open 11;7 (2018).
5. Someya, Y. et al. Investigation of Breast Cancer Microstructure and Microvasculature from Time-Dependent DWI and CEST in Correlation with Histological Biomarkers. in submission (2021).
6. Britto, D. D. et al. Macrophages enhance Vegfa-driven angiogenesis in an embryonic zebrafish tumour xenograft model. Dis. Model. Mech. 11(12) (2018).
Figures