4276
Breast amide proton transfer imaging at 3T: diagnostic performance and correlation with pathologic characteristics1National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital & Shenzhen Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Shenzhen, China, 2MR Research, GE Healthcare, Beijing, China, 3Paul C. Lauterbur Research Center for Biomedical Imaging, Shenzhen Institute of Advanced Technology, Chinese Academy of Sciences, Shenzhen, China
Synopsis
To date, great efforts have been made to investigate the performance of APT imaging in breast cancer diagnosis and prognosis, but with discrepant findings. Hence, this study aims to investigate the diagnostic performance and association of APT imaging with clinical-pathologic characteristics of breast cancer in a relatively large cohort of patients at 3T. Results showed significantly higher APTw signals in malignant lesions than that in benign ones, and high histologic grade, T stage and proliferation Ki-67 index in breast cancer patients. The results confirm the usefulness of APT imaging in the differentiation of lesion malignancy and the prediction of prognosis.
Introduction
As one of the most common cancers worldwide, accounting for almost 30% of cancers in female population with a high mortality-to-incidence ratio of 15% [1], breast cancer is composed of highly heterogeneous tumors with various histologic subtypes and grades, molecular hallmarks and subtypes, gene mutation, and immunomarkers [2], leading to a variety of different tailored treatment schedules. Non-invasive imaging methods capable of revealing such histologic and molecular characteristic heterogeneity are desired to guide individualized therapeutic strategy. To date, great efforts have been made to investigate the performance of APT imaging in breast cancer diagnosis and prognosis, but with discrepant findings. Hence, this study aims to investigate the diagnostic performance and association of APT imaging with clinical-pathologic characteristics of breast cancer in a relatively large cohort of patients at 3T, which may provide supplementary information for better elucidation of the feasibility of APT imaging in guiding clinical treatment of breast cancer.Materials and Methods
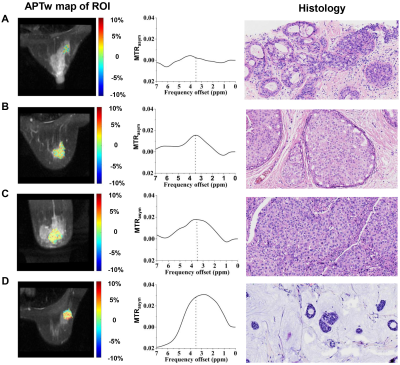
This retrospective study enrolled 84 patients with pathologically confirmed breast lesions who had undergone APT imaging before treatment at 3T. Chemical shift-selective fat suppression and single-shot fast spin echo (FSE) readout were used (4 radiofrequency saturation pulses with duration of 500 ms for each pulse and without inter-pulse delay, RF saturation power B1 of 2 μT, TR = 6189 ms with minimum TE, slice thickness = 4 mm, field of view = 12×12 cm2, matrix size = 96×96). In addition to an unsaturated scan, 27 CEST-weighted images with frequency offsets of -7 to 7 ppm in 0.5 ppm increments were acquired. To correct B0 inhomogeneity, a water saturation shift referencing (WASSR) map was collected with B1 of 0.5 μT (frequency offsets including ±240, ±192, ±144, ±96, ±48 and 0 Hz). APTw signal was quantified with magnetization transfer asymmetry analysis. Breast lesion was manually segmented, where averaged APTw signal was measured for each patient. Clinical-pathologic characteristics, including lesion malignancy, clinical T stage, histologic grades, Ki-67 proliferation index, molecular biomarkers (e.g., ER, PR, and HER-2), molecular subtypes (e.g., Luminal A, Luminal B, triple negative, and HER-2 enriched subtypes), and status of perineural and vascular invasion, were determined from histopathology. Student t-test, one-way analysis of variance, receiver operative characteristic analysis, and Pearson’s correlation were used for statistical analyses.Results and Discussions
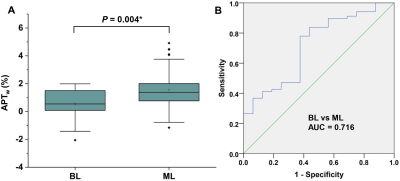
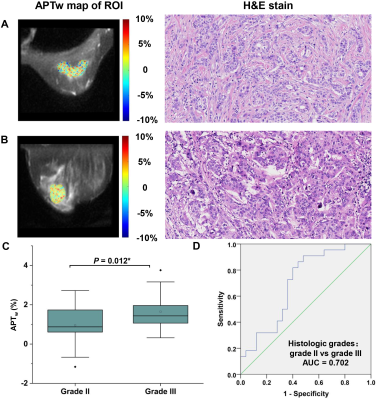
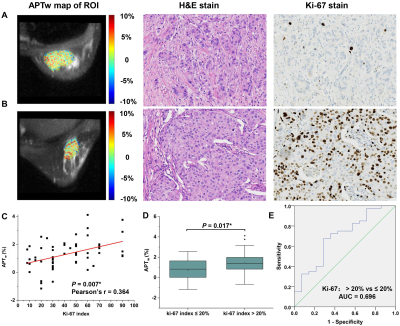
APTw signal was significantly higher in malignant lesions than that in benign lesions (1.55 ± 1.24 vs. 0.54 ± 1.13, P = 0.004), showing a sensitivity of 77.9%, specificity of 62.5% and area under the curve of 0.716 in discriminating the two lesion types (Figure 1&2), implying more active protein synthesis present in malignant lesions and suggesting the additional potential of using APT imaging in discriminate benign and malignant lesions. The clinical assessment of histologic grade for breast cancer comprises the assessment of glandular/tubular differentiation, nuclear pleomorphism, and mitotic activity, which are representative characteristics of tumor aggressiveness. For invasive breast carcinoma of no special type patients (IBC-NST), APTw signal was significantly higher in grade III lesions than grade II lesions (1.65 ± 0.84% vs. 0.96 ± 0.96%, P = 0.012) (Figure 3), suggesting the ability of APTw signal in noninvasively evaluating the histologic malignancy grade. Similarly, IBC-NST patients in T2 stage (1.57 ± 0.64%, P = 0.032) and T3 stage (1.54 ± 0.63%, P = 0.024) presented significantly higher APTw signals than those in T1 stage (0.81 ± 0.64%). Additionally, APTw signal showed significantly positive correlation with Ki-67 proliferation index (r = 0.364, P = 0.007) (Figure 4), a widely used and well-established immune-marker for assessing tumor proliferation. All these findings implied the potential of APT imaging in prognosis prediction of breast cancer. In contrast, no significant difference in APTw was found between groups with different status of ER, PR, HER-2, or perineural and vascular invasion, respectively, nor among the four molecular subtypes of Luminal A, Luminal B, triple negative, and HER-2 enriched (all P > 0.05), implying the limited value of APT imaging in non-invasively predicting molecular hallmarks of breast cancer. Note that although this study showed limited value of APT imaging in predicting the status of perineural and vascular invasion in the primary breast lesions for the first time, further in-depth study of this issue is still necessary in the future.Conclusions
APT imaging enables the identification of lesion malignancy and the prediction of histologic grade, T stage, and proliferative activity of breast tumors, which may benefit treatment decisions and individualized care.Acknowledgements
Special thanks to Long Qian from GE healthcare for his technical support.References
1. Siegel RL, Miller KD, Jemal A (2020) Cancer statistics, 2020. CA Cancer J Clin 70:7–30
2. Loibl S, Poortmans P, Morrow M, et al (2021) Breast cancer. Lancet 397:1750–1769
Figures