4272
Differentiating malignant and benign breast tumors using a continuous-time random-walk diffusion model1Medical Imaging Center, Shenzhen Hospital, Southern Medical University, Shenzhen, China, 2United Imaging Healthcare, Shanghai, China, 3Shanghai Key Laboratory of Magnetic Resonance, Department of Physics, East China Normal University, Shanghai, China
Synopsis
Magnetic resonance imaging (MRI) is an important diagnostic method for the breast cancer. However, for conventional MRI techniques such as diffusion-weighted imaging (DWI), it ignores the non-Gaussian behaviors of the water diffusion caused by the restriction of tissue microstructure, thus the conventional method is insensitive to microstructural and heterogeneity changes in breast tumor. This study explores the feasibility of applying a continuous-time random-walk (CTRW) non-Gaussian diffusion model to the differentiation and assessment of malignant and benign breast tumors. The correlations between CTRW parameters and breast tumor immunohistochemical features such as oestrogen receptor (ER), progesterone receptor (PR) and Ki67 expression status are studied. The CTRW model has potential in future applications of breast tumor malignancy and therapeutic efficacy evaluation.
Introduction
Breast cancer is the most common malignant cancer and the leading cause of cancer mortality among females worldwide[1, 2]. The timely and precise measurement of the breast tumor as well as its biological markers is of great importance in cancer diagnosis and its treatment, which can yield better prognosis and higher survival rate[2].Magnetic resonance imaging (MRI) outperforms conventional breast tumor diagnostic methods such as breast biopsy and digital mammography since it is non-invasive, ionizing radiation-free, and with high imaging resolution[3]. In particular, diffusion-weighted imaging (DWI), which can probe the underlying tissue structures in vivo, has been widely used in breast tumor evaluation[4-6]. However, as a simple mono-exponential model, conventional DWI model based on the assumption that water diffusion follows a Gaussian behavior, while the diffusion is normally restricted by tissue microstructure and shows non-Gaussian phenomena in living tissue[7]. Recently, a continuous-time random-walk (CTRW) diffusion model was raised and found to be sensitive to microstructural and heterogeneity changes in tumor tissues[7-9]. In this study, we aimed to apply CTRW diffusion model to differentiate malignant and benign breast tumors, and to investigate the correlation between tumor immunohistochemical biomarkers and CTRW-derived parameters.
Methods
From June 2021 to October 2021, 31 patients with breast tumor (12 malignant and 19 benign) were enrolled into this prospective study with approval of the institutional ethic committee. All the MRI examinations were carried out with a commercial 3.0 T scanner (uMR 790, United Imaging Healthcare, Shanghai, China). DWI was conducted on an echo planner imaging (EPI) sequence with following parameters: TE/TR/Flip angle: 80.1 ms/ 3897 ms/ 90°, matrix: 104 × 192, field of view: 190 mm × 350 mm, 27 slices, slice thickness: 4 mm, b-values = 0, 10, 20, 50, 80, 100, 200, 400, 600, 800, 1000, 2000, 3000 s/mm2). The multi-b-value DWI datasets were analyzed using a CTRW model that yielded three parameters: temporal diffusion heterogeneity α, spatial diffusion heterogeneity β, and an anomalous diffusion coefficient Dm[7, 8]. The molecular prognostic biomarkers of breast cancer, including Ki67, estrogen receptor (ER), and progesterone receptor (PR) were recorded[4].Statistical analyses were performed using the GraphPad PRISM 6 (Version 6.01; GraphPad Software, Inc., La Jolla, USA). The independent-sample t-test was used to compare the differences of CTRW parameters between the benign and malignant breast tumor groups. The same method was also used to compare the differences of CTRW parameters between the Ki67 high and low expression groups. Spearman’s correlation coefficient was used to quantify the correlations between each CTRW model parameter and breast tumor immunohistochemical biomarkers. All statistical tests were two-tailed and a P value < 0.05 was considered statistically significant.
Results
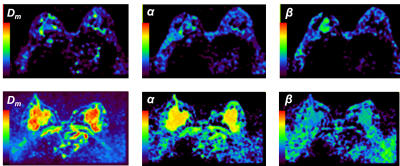
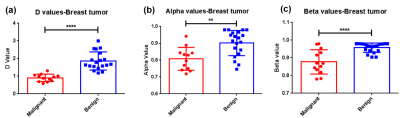
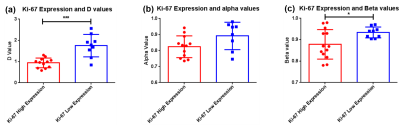
The CTRW parameter maps of a typical patient with malignant breast tumor and another typical patient with benign breast tumor are shown in Figure 1, with Dm parameter on the first column, α parameter on the second column and the β on the third column. For the malignant breast tumor subgroup, the calculated Dm, α and β parameters are significantly lower than those corresponding parameters of benign breast tumor subgroup (Dm : 0.905 ± 0.061 vs 1.862 ± 0.118 μm2/s, ****P < 0.0001; α: 0.808 ± 0.020 vs 0.902 ± 0.017, **P = 0.001; β: 0.877 ± 0.019 vs 0.957 ± 0.006, ****P < 0.0001), as shown in Figure 2.In breast tumors with high Ki67 expression (> 15 %)[10], it is found that the CTRW model parameters Dm and β are significantly lower than the corresponding parameters of breast tumors with low Ki67 expression (≤ 15%)[10] (Dm: 0.939 ± 0.066 vs 1.753 ± 0.176, ***P = 0.0001; β: 0.878 ± 0.019 vs 0.934 ± 0.009, *P = 0.033), which is shown in Figure 3. Although for α parameter there is no significant difference between the above two groups, there is still a decrease of α parameter in breast tumors with high Ki67 expression.
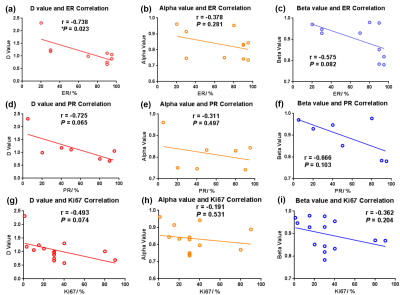
By studying the correlation between each CTRW model parameter and breast tumor immunohistochemical features including the oestrogen receptor (ER), progesterone receptor (PR) and Ki67 expression status respectively, it is found that in general, the CTRW model parameters exhibit a linear negative correlation with the measured expression level of ER, PR and Ki67, respectively, as shown in Figure 4. Among them, the parameter Dm shows a significant negative correlation with ER expression (r = -0.738, *P = 0.023).
Discussion
Despite DWI with monoexponential model had been widely used in breast tumor diagnosis, it ignored the property of tumor heterogeneity since it assumed a homogeneous diffusion process within the tumor. By contrast, non-Gaussian diffusion model might be more effective in reflecting the complex diffusion process within the tumor tissue.There are significant differences in the CTRW parameters between the malignant and benign tumor groups. The malignant tumors have a significant lower α and β than in the benign tumors, which is caused by the severe spatiotemporal diffusion heterogeneities in the malignant tumor due to the presence of necrosis, cyst and hemorrhage[11]. The anomalous diffusion coefficient Dm has a similar role to ADC and thus can be sensitive to tissue cellularity[8].
A higher Ki67 expression is correlated with higher tumor cell proliferation and a poorer prognosis[12, 13]. The present study found that high Ki67 expressed breast lesions have a lower Dm and β, this may due to the rapid tumor proliferation and an insufficient blood supply, leading to high cellular density and greater tissue necrosis. (As a result, it is not conducive to the diffusion of water molecules and increases the complexity of the tissue structure.)
Previously, Lei Tang et al reported that in a high-grade brain tumor medulloblastoma patient, the CTRW model parameters Dm, α, and β were significantly lower than the surrounding normal tissue[7]. Their group claim that multiparametric Dm, α, and β map can provide critical complementary information to the conventional T1, T2, and FLAIR contrast maps[7], thus can be further used to improve the accuracy and efficiency of cancer detection.
The CTRW model parameters exhibit a linear negative correlation with the measured expression level of ER, PR and Ki67, respectively. Currently, only the parameter Dm shows a significant negative correlation with ER expression. This may due to the limited number in each ER, PR and Ki67 subgroup since in some patients, the above immunohistochemical information is partially incomplete. In the future study, the corresponding biomarker information will be collected and analyzed.
Conclusion
The CTRW diffusion MR model derived parameters Dm, α and β vary significantly according to the malignant and benign status of breast tumor and its immunohistochemical biomarkers. This model has potential in future applications of breast tumor malignancy assessment, evaluation of therapeutic efficacy and management of long-term prognosis.Acknowledgements
No acknowledgement found.References
[1] Waks A G, Winer E P. Breast cancer treatment: A review [J]. JAMA, 2019, 321(3): 288-300.
[2] Sun Y-S, Zhao Z, Yang Z-N, et al. Risk factors and preventions of breast cancer [J]. International journal of biological sciences, 2017, 13(11): 1387.
[3] Akram M, Iqbal M, Daniyal M, et al. Awareness and current knowledge of breast cancer [J]. Biological research, 2017, 50(1): 1-23.
[4] Martincich L, Deantoni V, Bertotto I, et al. Correlations between diffusion-weighted imaging and breast cancer biomarkers [J]. European radiology, 2012, 22(7): 1519-1528.
[5] Woodhams R, Matsunaga K, Iwabuchi K, et al. Diffusion-weighted imaging of malignant breast tumors: The usefulness of apparent diffusion coefficient (adc) value and adc map for the detection of malignant breast tumors and evaluation of cancer extension [J]. Journal of computer assisted tomography, 2005, 29(5): 644-649.
[6] Kuroki Y, Nasu K. Advances in breast mri: Diffusion-weighted imaging of the breast [J]. Breast cancer, 2008, 15(3): 212-217.
[7] Tang L, Zhou X J. Diffusion mri of cancer: From low to high b‐values [J]. Journal of Magnetic Resonance Imaging, 2019, 49(1): 23-40.
[8] Karaman M M, Sui Y, Wang H, et al. Differentiating low-and high-grade pediatric brain tumors using a continuous-time random-walk diffusion model at high b-values [J]. Magnetic resonance in medicine, 2016, 76(4): 1149-1157.
[9] Ingo C, Magin R L, Colon‐Perez L, et al. On random walks and entropy in diffusion‐weighted magnetic resonance imaging studies of neural tissue [J]. Magnetic resonance in medicine, 2014, 71(2): 617-627.
[10] Inwald E, Klinkhammer-Schalke M, Hofstädter F, et al. Ki-67 is a prognostic parameter in breast cancer patients: Results of a large population-based cohort of a cancer registry [J]. Breast cancer research and treatment, 2013, 139(2): 539-552.
[11] Shimizu H, Kumabe T, Shirane R, et al. Correlation between choline level measured by proton mr spectroscopy and ki-67 labeling index in gliomas [J]. American Journal of Neuroradiology, 2000, 21(4): 659-665.
[12] Fan M, Yuan W, Zhao W, et al. Joint prediction of breast cancer histological grade and ki-67 expression level based on dce-mri and dwi radiomics [J]. IEEE journal of biomedical and health informatics, 2019, 24(6): 1632-1642.
[13] Shin J K, Kim J Y. Dynamic contrast‐enhanced and diffusion‐weighted mri of estrogen receptor‐positive invasive breast cancers: Associations between quantitative mr parameters and ki‐67 proliferation status [J]. Journal of Magnetic Resonance Imaging, 2017, 45(1): 94-102.
Figures