4271
Potential of a Nomogram Based on DCE MRI and Clinical Features to Reduce Unnecessary Biopsies of Breast Lesions: Comparison with BI-RADS1Department of Radiology, The First Affiliated Hospital of Wenzhou Medical University, Wenzhou, China, 2Department of Radiological Sciences, University of California, Irvine, CA, United States
Synopsis
318 patients (331 breast lesions) with DCE-MRI and clinical features were analyzed to establish a nomogram for diagnosis of breast cancer. The dataset was split to 233 (145 malignant 88 benign) for training, and 98 (61 malignant 37 benign) for testing. Radiomics features were extracted from DCE-MRI and selected to calculate the radiomics score. The clinical features were analyzed by univariate and multivariate analyses. Then a nomogram was established based on clinical features and rad-score. When applying cut-off values with ≥95% sensitivity, the nomogram can reduce 59.5% to 65.9% unnecessary biopsies, which was higher than that of BI-RADS.
Introduction:
Although MRI has the highest sensitivity for breast cancer detection among the current clinical imaging modalities, its specificity is still insufficient. The evaluation is mainly based on visual assessments by radiologists, which might be affected by the variability of observers lead to unnecessary biopsies. Radiomics analysis has been developed in recent years, which extract quantitative features not recognized by the naked eye to develop models for different clinical applications in precision oncology 1-4. In addition, evidence has shown that breast cancer is a complex disorder affected by multiple risk factors such as age, menopausal status, BMI, and genetics 5-7. Combination of clinical information, blood test results, and imaging findings may improve the diagnostic performance of breast cancer. We aimed to establish a nomogram based on DCE-MRI radiomics, clinical, and blood biochemical test results for differential diagnosis of benign and malignant lesions, and compared with Breast Imaging Report and Data System (BI-RADS).Methods:
We retrospectively analyzed 318 patients with 331 pathologically confirmed breast lesions, of which 125 were benign and 206 were malignant. These lesions were randomly divided into the training set and the validation set according to a ratio of 7:3. All patients underwent DCE-MRI scans and blood biochemical examination before biopsy or surgery. A total of 3,948 radiomics features were extracted from lesions in signal enhancement ratio map, maximum slope of increase map, and maximum slope of decrease map. After feature selection by the minimum redundancy maximum relevance and the least absolute shrinkage and selection operator methods, a radiomics score (rad-score) was calculated by summing the selected features weighted by their coefficients. The clinical features were analyzed by univariate and multivariate analyses and used to build a clinical model. Logistic regression was used to establish a nomogram based on clinical features and rad-score. The diagnostic performances of all models were assessed by area under the receiver operator characteristic curve (AUC) and compared by the DeLong test. The McNemar test was performed to compare the potential to help reduce unnecessary biopsies between various models and BI-RADS at a high sensitivity level (≥95%).Result:
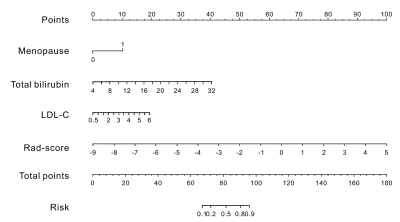
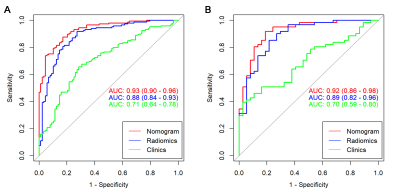
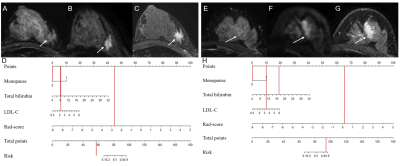
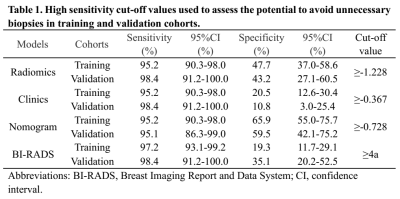
After feature selection, there were 11 radiomics features remained for calculation of rad-score. In the multivariate analysis, menopause status, total bilirubin (TB) and low-density lipoprotein-cholesterol (LDL-C) were risk factors for breast cancer. A combination model was built based on rad-score, menopause status, TB and LDL-C, and displayed in the form of a nomogram (Fig.1). The ROC curves of the clinical model, radiomics model, and nomogram are shown in Fig.2. The nomogram yielded an AUC of 0.93 [95% confidence interval (CI), 0.90-0.96] in the training cohort and an AUC of 0.92 (95% CI, 0.86-0.98) in the validation cohort. Two examples of the nomogram in use are shown in Fig.3. When applying cut-off values with ≥95% sensitivity, the nomogram can reduce 59.5% to 65.9% unnecessary biopsies, which was higher than that of BI-RADS (19.3% to 35.1%, p<0.001) (Table 1).Discussion:
In this study, we established a nomogram based on radiomics of DCE-MRI and clinical, biochemical blood test features. The result demonstrated that the nomogram had excellent diagnostic ability for breast cancer diagnosis and potential to reduce unnecessary biopsies. The ability to reduce unnecessary biopsies of the nomogram was further improved to about 1.7 to 5.5 times that of BI-RADS, without sacrificing diagnostic sensitivity. Previous studies have confirmed that menopausal status and the level of LDL-C are correlated with the risk of breast cancer 8-10. Considering the multi-factors and complexity of breast cancer, integrating many-sided effective information of patients could contribute to a more efficient and accurate diagnosis and individualized treatment.Acknowledgements
This work was supported by funding from the Medical Health Science and Technology Project of Zhejiang Province Health Commission (2019326177) and the Wenzhou Municipal Science and Technology Bureau (Y20180185).References
1. Lambin P, Rios-Velazquez E, Leijenaar R, et al. Radiomics: extracting more information from medical images using advanced feature analysis. Eur J Cancer. 2012; 48(4):441-6.
2. Liu Z, Wang S, Dong D, et al. The Applications of Radiomics in Precision Diagnosis and Treatment of Oncology: Opportunities and Challenges. Theranostics. 2019; 9(5):1303-22.
3. Crivelli P, Ledda RE, Parascandolo N, Fara A, Soro D, Conti M. A New Challenge for Radiologists: Radiomics in Breast Cancer. Biomed Res Int. 2018; 2018:6120703.
4. Kim SG, Freed M, Leite APK, Zhang J, Seuss C, Moy L. Separation of benign and malignant breast lesions using dynamic contrast enhanced MRI in a biopsy cohort. J Magn Reson Imaging. 2017; 45(5):1385-93.
5. Iyengar NM, Gucalp A, Dannenberg AJ, Hudis CA. Obesity and Cancer Mechanisms: Tumor Microenvironment and Inflammation. J Clin Oncol. 2016; 34(35):4270-6.
6. Choi J, Cha YJ, Koo JS. Adipocyte biology in breast cancer: From silent bystander to active facilitator. Prog Lipid Res. 2018; 69:11-20.
7. Iyengar NM, Morris PG, Zhou XK, et al. Menopause is a determinant of breast adipose inflammation. Cancer prevention research (Philadelphia, Pa). 2015; 8(5):349-58.
8. Nowak C, Arnlov J. A Mendelian randomization study of the effects of blood lipids on breast cancer risk. Nature communications. 2018; 9(1):3957.
9. Jung YY, Kim HM, Koo JS. Expression of Lipid Metabolism-Related Proteins in Metastatic Breast Cancer. PloS one. 2015; 10(9):e0137204.
10. Cedo L, Reddy ST, Mato E, Blanco-Vaca F, Escola-Gil JC. HDL and LDL: Potential New Players in Breast Cancer Development. J Clin Med. 2019; 8(6).
Figures