4263
Can combined DWI, IVIM and DKI better differentiate malignant from benign breast lesions with TIC type II?1Clinical Medical College of Jining Medical University, Jining, China, 2Department of Medical Imaging, Affiliated Hospital of Jining Medical University, Jining, China, 3MR Research China, GE Healthcare, Beijing, China
Synopsis
Accurate preoperative prediction of benign and malignant breast lesions is essential in clinical practice. Currently, the diagnostic specificity of DCE-MRI is relatively low for breast lesions with time-intensity-curve (TIC) type-II. Different diffusion-weighted models, including mono-exponential diffusion-weighted imaging (DWI), intravoxel-incoherent-motion diffusion-weighted imaging (IVIM) and diffusion kurtosis imaging (DKI), have been previously reported effective in the differential diagnosis of overall breast lesions. In this study, we found that, compared with DWI alone, combined DWI, IVIM and DKI can better differentiate malignant from benign breast TIC type-II lesions, with IVIM parameter of D and DKI parameter of MK as demonstrated independent influencing factors.
Target audience
Researchers who are interested in the diagnosis of breast diseases and application of diffusion MRISummary of main findings
Compared with DWI alone, combined DWI, IVIM and DKI can better differentiate malignant from benign breast TIC type-II lesions, with IVIM parameter of D and DKI parameter of MK as demonstrated independent influencing factors.Introduction
Although more than half of breast cancer shows a type-III time-intensity-curve (TIC) in DCE-MRI, there are still about 34% of breast cancer demonstrating a type-II curve. Meanwhile, about 11.5% of benign breast lesions also present type-II curve1. So, there is an overlap between benign and malignant TIC type-II breast lesions, making the preoperative diagnosis challenging2.Mono-exponential DWI with apparent diffusion coefficient (ADC) is routinely used to diagnose different types of breast lesions3, although microcirculation perfusion is not taken into account in this model. Intravoxel-incoherent-motion DWI (IVIM), a multiple b value DWI, allows bi-exponential model for assessing tissue diffusion and perfusion information simultaneously4. With this technique, the diagnostic performance of D value , one of IVIM parameters, was superior to that of the ADC value in the preoperative identification of breast tumors5. However, both DWI and IVIM might not assess the water molecules in breast lesion accurately with only considering Gaussian water diffusion. In comparison, diffusion kurtosis imaging (DKI) was developed with assumption of non-Gaussian distributed diffusion of water molecules and may thus more truly evaluate the diffusion of water molecules in breast tumors6. With individual features, these three diffusion models of DWI, IVIM and DKI, however, have not been combined to differentiate malignant from benign TIC type-II breast lesions so far.
Therefore, in this study we aimed to explore the clinical potential of combing these three diffusion models in improving the diagnostic accuracy of different types of TIC type-II breast lesions.
Materials and Methods
103 patients (103 female, mean age: 49), who underwent preoperative DWI, IVIM and DKI and had TIC type-II breast lesions confirmed by pathology, were reviewed in this study retrospectively. Informed consent was obtained from each patient.Patients were divided into benign group (25 lesions in 25 patients, including 16 fibroadenoma, 5 adenopathy, 2 abscesses, 1 Cyst, and 1 Cellular neurofibroma) and malignant group (78 lesions in 78 patients, including 68 invasive carcinomas, 4 carcinomas in situ, 2 intra-cystic papillary carcinoma, 2 mucinous carcinoma, 1 Metaplastic cancer, 1 secretory carcinoma).
MRI imaging
MR examinations were performed in a 3.0T MR (Discovery 750W, GE healthcare) with 8-channel phased-array breast coil.IVIM (TR 2500ms, TE 90ms, FOV 350 mm×350 mm, matrix 128×128, b-values = 0, 20, 30, 50, 70, 100, 150, 200, 500, 700, 1000, 1500, 2000 sec/mm2, NEX = 2, scan time = 400 sec) and DKI (TR 5000 ms, TE 89.9 ms, FOV 350 mm×350 mm, matrix 128×128, b-values = 0, 1000, 2000 sec/mm2, diffusion direction per each b value of 30, NEX = 2, scan time = 355 sec) were performed using a single-shot 2D spin-echo echo-planar-imaging (EPI) sequence with fat saturation.
All relevant functional parametric maps of DWI, IVIM, and DKI were obtained by using GE AW4.6 workstation. Mono-exponential model derived ADC for DWI, bi-exponential model derived true diffusion coefficient (D), perfusion-related diffusion coefficient (D*), perfusion fraction (f) for IVIM, and non-gaussian diffusion model derived mean diffusion rate (MD) and mean kurtosis value (MK) for DKI, were obtained for each patient accordingly7. Two senior observers were employed for manual delineation of the region of interest (ROI) at the largest solid part of the tumor independently, avoiding necrosis, cystic and liquefaction areas by referring to DCE-MRI imaging. The averaged ROI was then overlaid on the other parameter maps.
Statistical Methods
Statistical analyses were performed using SPSS software version-26.0. Intraclass correlation coefficients (ICC) analysis was used to evaluate the inter-observer agreement. Independent samples t-test was used to compare the differences of each diffusion parameter between two groups. Logistic regression analyses were further performed to find independent influencing factors. Receiver operating characteristic curve (ROC) and area under the curve (AUC) were separately used to analyze the efficacy of each diffusion parameter and in combination in differentiating malignant from benign breast TIC type-II lesions.Results
Excellent inter-observer agreement of each diffusion parameter measurement was confirmed by high ICC values (all>0.75).Lower ADC, D, and MD and higher f and MK values were found in malignant than benign breast lesions (1.56±0.49 vs. 1.06±0.31, 1.29±0.45 vs. 0.81±0.2, 3.17±1.01 vs. 2.18±0.67, P < 0.001; 0.41±0.19 vs. 0.32±0.15, P =0.030; 0.46±0.12 vs. 0.73±0.21, P < 0.001; Figs. 1 and 2).
With logistic regression analysis, each diffusion parameter showed a significant effect on differential diagnosis of malignant and benign breast TIC type-II lesions. D and MK were considered independent influencing factors with the largest odds ratio for MK (Table 1).
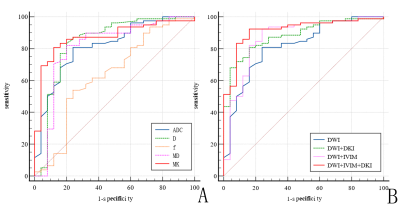
Using ROC analysis, combined three diffusion models achieved the greatest diagnostic efficiency (AUC 0.915, sensitivity 92.3%, and specificity 84.0%) (Table 2, Fig. 3).
Discussion and Conclusion
Compared with DWI derived ADC, combined DWI, IVIM and DKI can better differentiate malignant from benign breast TIC type-II lesions. IVIM parameter of D and DKI parameter of MK were shown as independent influencing factors.In conclusion, combined DWI, IVIM and DKI has been demonstrated as a robust tool in differential diagnosis of benign and malignant breast TIC type-II lesions.
Acknowledgements
No acknowledgement found.References
1. Kuhl C K, Mielcareck P, Klaschik S, et al. Dynamic breast MR imaging: are signal intensity time course data useful for differential diagnosis of enhancing lesions? [J]. Radiology, 1999, 211(1): 101-110.
2. Thakran S, Gupta P K, Kabra V, et al. Characterization of breast lesion using T1-perfusion magnetic resonance imaging: Qualitative vs. quantitative analysis [J]. Diagn Interv Imaging, 2018, 99(10): 633-642.
3. Yang X, Dong M, Li S, et al. Diffusion-weighted imaging or dynamic contrast-enhanced curve: a retrospective analysis of contrast-enhanced magnetic resonance imaging-based differential diagnoses of benign and malignant breast lesions [J]. Eur Radiol, 2020, 30(9): 4795-4805.
4. Li K, Machireddy A, Tudorica A, et al. Discrimination of Malignant and Benign Breast Lesions Using Quantitative Multiparametric MRI: A Preliminary Study [J]. Tomography, 2020, 6(2): 148-159.
5. Liang J, Zeng S, Li Z, et al. Intravoxel Incoherent Motion Diffusion-Weighted Imaging for Quantitative Differentiation of Breast Tumors: A Meta-Analysis [J]. Front Oncol, 2020, 10(585486.
6. He M, Ruan H, Ma M, et al. Application of Diffusion Weighted Imaging Techniques for Differentiating Benign and Malignant Breast Lesions [J]. Front Oncol, 2021, 11(694634.
7. You C, Li J, Zhi W, et al. The volumetric-tumour histogram-based analysis of intravoxel incoherent motion and non-Gaussian diffusion MRI: association with prognostic factors in HER2-positive breast cancer [J]. J Transl Med, 2019, 17(1): 182.
Figures
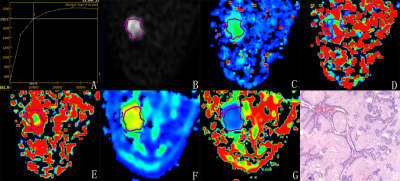
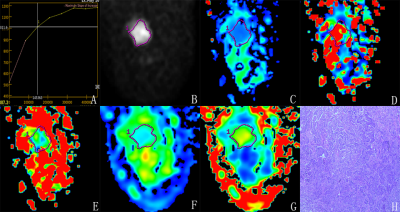
Figure 2 A 52-year-old female with mass in lower-out quadrant of the right breast. TIC of the mass is type-II (A). The ADC value is 0.65×10-3 mm2/s (B), the D value is 0.402×10-3 mm2/s (C), the D* value is 84.0×10-3 mm2/s (D), the f value is 8.36% (E), the MD value is 1.26×10-3 mm2/s (F), and the MK value is 1.21 (G). The pathological diagnosis(magnification =×40) is invasive ductal carcinoma (H).
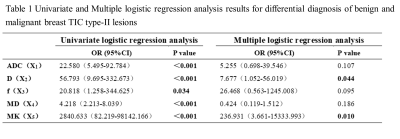
Table 1 Univariate and Multiple logistic regression analysis results for differential diagnosis of benign and malignant breast TIC type-II lesions
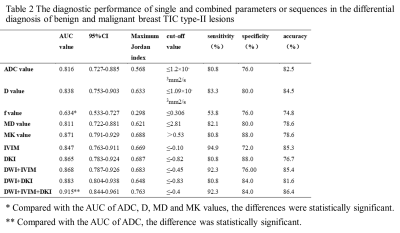
Table 2 The diagnostic performance of single and combined parameters or sequences in the differential diagnosis of benign and malignant breast TIC type-II lesions