4260
Optimal breath-hold dynamic liver MRI using a combination of compressed sensing with contrast-enhanced timing robust angiography technique
Nobuyuki Kawai1, Yoshifumi Noda1, Tetsuro Kaga1, Kimihiro Kajita2, and Masayuki Matsuo1
1Gifu University, Gifu, Japan, 2Gifu University Hospital, Gifu, Japan
1Gifu University, Gifu, Japan, 2Gifu University Hospital, Gifu, Japan
Synopsis
Rapid dynamic liver MRI with compressed-sensing sensitivity encoding (Compressed SENSE) can reduce respiratory motion artifacts. However, Since the central k-space filling is random or posterior half of the acquisition in Compressed SENSE, optimal acquisition timing is missed only using a conventional scan delay method for hepatic arterial dominant phase (HAP). We assessed a combination of Compressed SENSE with contrast enhanced robust angiography (CENTRA) for dynamic liver MRI. CS-CENTRA sequence achieved significantly higher arterial enhancement with comparable CNR compared to CS-conventional one in HAP. CS-CENTRA contributed to optimal acquisition timing for HAP.
Introduction
Gadoxetic acid-enhanced MRI is an essential imaging modality for assessing hepatic diseases. Further, it is recommended by several guidelines on hepatocellular carcinoma (HCC) 1-4. A bolus injection of gadoxetic acid can facilitate the assessment of tumor vascularity and hemodynamics via the hepatic arterial dominant phase (HAP) to portal venous phase. Moreover, it has an excellent tissue contrast for the differentiation between focal hepatic lesions and liver parenchyma in the hepatobiliary phase, which is obtained 15‒20 minutes after contrast administration 5.Gadoxetic acid-enhanced MRI is commonly performed with breath-hold sequence using fat-suppressed 3-dimentional gradient echo T1-weighted imaging 6. However, breath-hold imaging has become a problem for patients with compromised breath-hold capacity. In addition, recent report suggests that the administration of gadoxetic acid reduces breath-hold capacity in the hepatic arterial phase and causes transient severe motion artifacts 7. One of the solutions for this problem is to reduce acquisition time using rapid imaging technique such as compressed sensing (e.g. Compressed SENSE; compressed-sensing sensitivity encoding). Since its introduction for dynamic liver MRI, respiratory motion artifacts were significantly reduced 8.
Rapid dynamic liver MRI with Compressed SENSE can cause another problem. Since the central k-space filling is random or posterior half of the acquisition in Compressed SENSE, optimal acquisition timing is missed only using a conventional scan delay method for HAP 9. Here, contrast enhanced robust angiography (CENTRA) is centric k-space filling technique, which starts filling in the center of k-space and used mainly MR angiography 10. We hypothesized that a combination of Compressed SENSE with CENTRA for dynamic liver MRI contributed to optimal acquisition timing for HAP. The purpose of this study was to evaluate the feasibility of a combination of Compressed SENSE with CENTRA for dynamic liver MRI.
Materials and Methods
This retrospective HIPAA-compliant study was approved by our Institutional Review Board, and written informed consent was waived. Forty-five patients underwent gadoxetic acid-enhanced dynamic imaging using a combination of Compressed SENSE with CENTRA (CS-CENTRA; 29 men and 16 women; mean age, 68 years; age range, 37‒88 years) and were compared with propensity score-matched 45 patients who underwent conventional imaging with Compressed SENSE (CS-conventional; 28 men and 17 women; mean age, 67 years; age range, 11‒89 years) at a 3-T scanner with a 32-channel phased-array receiver coil. Propensity score matching was based on patients’ age, gender, and body mass index. After obtaining precontrast images, 0.1 mL/kg of gadoxetic acid was administered at a rate of 2 mL/s followed by a 30 mL saline flush at the same rate. Subsequently, HAP and portal venous phase were obtained at 5 s and 45 s after arrival of the contrast agent at abdominal aorta detected by fluoroscopic bolus tracking system; and transitional phases were subsequently obtained at 120 s and 180 s after administration of the contrast agent. Hepatobiliary phase images were obtained during 15‒21 min after an intravenous bolus injection of gadoxetic acid. All injections were performed using a commercially available power injector. Acquisition time in HAP was 11 s both for CS-CENTRA and for CS-conventional group, however, central k-space filling time (k = 0) was approximately 3.6 s and 7.8 s delay from start of the scan, respectively (Table 1). For quantitative image analyses, signal intensity ratio (SIR) of abdominal arteries, portal vein, and liver and pancreatic parenchyma were calculated in HAP. In addition, contrast-to-noise ratio (CNR) of HCC and pancreatic ductal adenocarcinoma (PDAC) were also calculated. For qualitative image analyses, a radiologist graded the degree of arterial enhancement in HAP using a five-point rating scale. The degrees of liver and pancreatic parenchymal enhancement were classified into three categories corresponding to early, appropriate, and late arterial phase (AP). Unpaired t-test for quantitative measurements, and Mann-Whitney U test or Fisher’s exact test for qualitative scales were performed to evaluate differences between the two groups.Results
Quantitative results were demonstrated in Table 2. SIRs of aorta, celiac artery, proper hepatic artery, splenic artery, and superior mesenteric artery in CS-CENTRA group were significantly higher than those in CS-conventional group (P = 0.005‒0.024). There were no significant differences in SIRs of common hepatic artery, portal vein, and liver and pancreatic parenchyma, and in CNRs of HCC and PDAC between the two groups (P = 0.132‒0.556). The degree of arterial enhancement in CS-CENTRA (4.0) was comparable with that in CS-conventional (3.6) (P = 0.129). Categorical distribution of liver and pancreatic parenchymal enhancement was summarized in Table 3. The ratios of appropriate AP in liver and pancreatic parenchyma in CS-CENTRA (75.6%, 93.3%, respectively) was higher than those in CS-conventional (60%, 71.1%, respectively).Discussion
CS-CENTRA sequence achieved significantly higher SIR of abdominal artery and comparable CNR for HCC and PDAC compared to CS-conventional one in HAP. Our results demonstrated that a combination of Compressed SENSE with CENTRA contributed to optimal acquisition timing for a conventional scan delay method for HAP. In addition, the ratios of appropriate AP in liver and pancreatic parenchymal enhancement in CS-CENTRA was higher than those in CS-conventional, which is important for accurate diagnostic imaging.Conclusion
CS-CENTRA sequence achieved significantly higher arterial enhancement with comparable CNR compared to CS-conventional one in HAP. CS-CENTRA contributed to optimal acquisition timing for HAP.Acknowledgements
The authors of this abstract declare no relationships with any companies whose products or services may be related to the subject matter of the article.References
- Omata M, Cheng AL, Kokudo N, et al. Asia-Pacific clinical practice guidelines on the management of hepatocellular carcinoma: a 2017 update. Hepatology international 2017;11:317-70.
- Kudo M, Matsui O, Izumi N, Iijima H, Kadoya M, Imai Y. Surveillance and diagnostic algorithm for hepatocellular carcinoma proposed by the Liver Cancer Study Group of Japan: 2014 update. Oncology 2014;87 Suppl 1:7-21.
- Marrero JA, Kulik LM, Sirlin CB, et al. Diagnosis, Staging, and Management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology (Baltimore, Md) 2018;68:723-50.
- EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. Journal of hepatology 2018;69:182-236.
- Sano K, Ichikawa T, Motosugi U, et al. Imaging study of early hepatocellular carcinoma: usefulness of gadoxetic acid-enhanced MR imaging. Radiology 2011;261:834-44.
- Goshima S, Noda Y, Kajita K, et al. Gadoxetic acid-enhanced high temporal-resolution hepatic arterial-phase imaging with view-sharing technique: Impact on the LI-RADS category. European journal of radiology 2017.
- McClellan TR, Motosugi U, Middleton MS, et al. Intravenous Gadoxetate Disodium Administration Reduces Breath-holding Capacity in the Hepatic Arterial Phase: A Multi-Center Randomized Placebo-controlled Trial. Radiology 2017;282:361-8.
- Kawai N, Goshima S, Noda Y, et al. Gadoxetic acid-enhanced dynamic magnetic resonance imaging using optimized integrated combination of compressed sensing and parallel imaging technique. Magnetic resonance imaging 2019;57:111-7.
- Goshima S, Kanematsu M, Kondo H, et al. Optimal acquisition delay for dynamic contrast-enhanced MRI of hypervascular hepatocellular carcinoma. AJR American journal of roentgenology 2009;192:686-92.
- Mende KA, Froehlich JM, von Weymarn C, et al. Time-resolved, high-resolution contrast-enhanced MR angiography of dialysis shunts using the CENTRA keyhole technique with parallel imaging. Journal of magnetic resonance imaging : JMRI 2007;25:832-40.
Figures
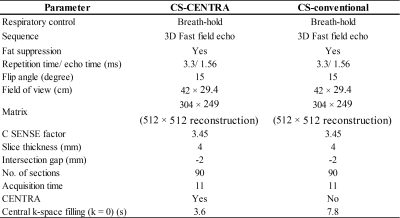
Table 1.
Scan parameters in CS-CENTRA and CS-conventional group.
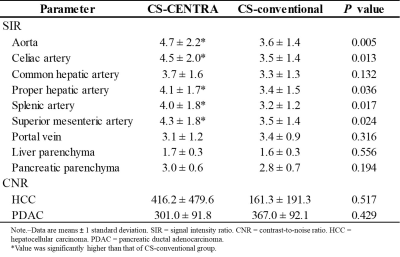
Quantitative measurements in CS-CENTRA and CS-conventional group.
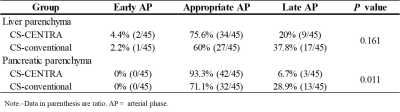
Table 3. Categorical distribution of liver and pancreatic parenchyma enhancement
in CS-CENTRA and CS-conventional group.
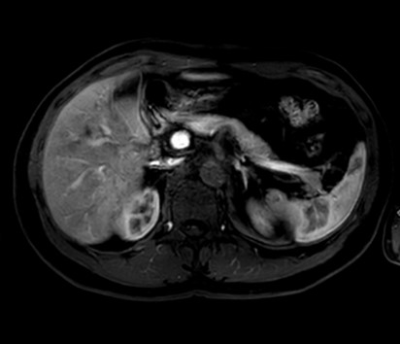
Fig
1. A 43-year-old male with postoperative state of rectal cancer in
CS-conventional group. Hepatic arterial phase image showed no enhancement of
aorta and celiac branches and marked enhancement of portal vein and hepatic
parenchyma, which was late for optimal arterial phase.
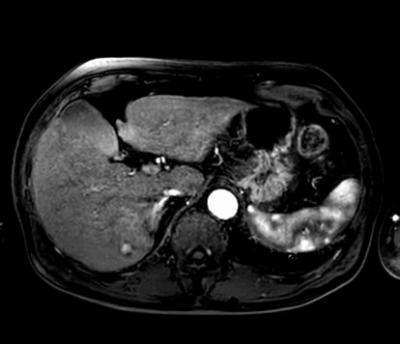
Fig
2. A 80-year-old male with non-alcoholic steatohepatitis in CS-CENTRA group. Hepatic
arterial phase image showed marked enhancement of aorta and celiac branches and
minimal to moderate enhancement of portal vein and hepatic parenchyma, which
was appropriate for optimal arterial phase. In addition, HCC was clearly depicted
in segment Ⅵ.
DOI: https://doi.org/10.58530/2022/4260