4252
Predicting treatment response using intratumoral and peritumoral radiomics based on CE-MRI for patients with HCC treated with TACE1The First Affiliated Hospital of Dalian Medical University, Dalian, China, 2Dalian Engineering Research Center for Artificial Intelligence in Medical Imaging, Dalian, China, 3Chengdu Institute of Computer Application, Chinese Academy of Sciences, Chengdu, China, 4University of Chinese Academy of Sciences, Beijing, China, 5Department of Radiology, The First Affiliated Hospital of Dalian Medical University, Dalian, China, 6GE Healthcare (China), Beijing, China
Synopsis
This work aimed at exploring the role of radiomics assessment of hepatocellular carcinoma (HCC) intratumoral and peritumoral regions based on contrast-enhanced magnetic resonance imaging (CE-MRI) to predict treatment response to transarterial chemoembolization (TACE) in patients with HCC. The results showed that the radiomics approach based on intratumoral and peritumoral regions has potential to improve performance for response prediction using preoperative CE-MRI.
Purpose
To develop and validate radiomics models based on peritumoral and intratumoral combined peritumoral derived from contrast-enhanced magnetic resonance imaging (CE-MRI) to predict the early therapeutic response of transarterial chemoembolization (TACE) in hepatocellular carcinoma (HCC).Introduction
Hepatocellular carcinoma (HCC) is the most common primary liver cancer and the fourth leading cause of death among various cancers [1]. Transarterial chemoembolization (TACE) is widely used as a bridge to liver transplantation, or as the mainstay of palliative treatment for patients with intermediate HCC [2]. Monitoring tumor response to TACE is a cornerstone in determining therapy efficacy, and plays a critical role in prognosis prediction and future treatment decision-making processes. MRI-based evaluations that are noninvasive and repeatable can be used to preoperatively assess the tumor response. Radiomics is an emerging methodology, with the goal of going beyond size or human-eye based semantic descriptors of tumors, to enable the non-invasive extraction of high-throughput quantitative data from medical images and to explore their correlation with clinical outcomes [3]. Recently, there has been increasing interest in evaluating radiomics patterns of the region surrounding the visible tumor [4,5]. Several scholars have reported that HCC patients with microvascular invasion (MVI) in peritumoral tissues have a significantly higher risk of recurrence or metastasis and cancer-related death [6]; thus, peritumoral tissues might have valuable predictive information of HCC prognosis. Therefore, we aimed at exploring the role of radiomics assessment of HCC intratumoral and peritumoral regions based on contrast-enhanced MR images to predict treatment response to TACE in patients with HCC.Materials and Methods
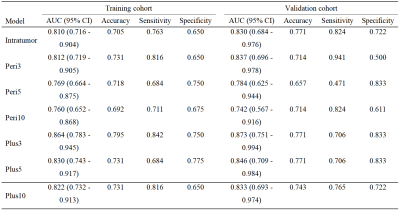
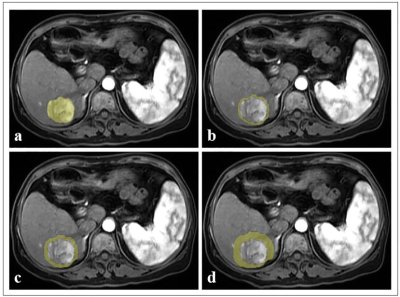
This retrospective study was approved by the Institutional Review Board. From April 2008 to August 2020, preoperative CE-MRI datasets of 113 HCC patients (55 objective response, 58 non-response) were collected and divided into the training (n = 78) and validation cohorts (n = 35). Tumor segmentation was performed by manually delineating the region of interest (ROI) along the tumor contour on each axial slice of arterial phase (AP), portal venous phase (PVP), and delayed phase (DP) images using an open-source software (ITK-SNAP, version 3.6.0, http://www.itksnap.org/). All of the AP, PVP and DP images and the delineated volumes of region (VOIs) were imported into the AK software. The VOIperi3, VOIperi5, and VOIperi10 were automatically generated by equidistant expansion of 3 mm, 5 mm and 10 mm from the lesion border, respectively (shown in Figure 1). Radiomics features were extracted using A.K. software for VOIintratumor, VOIperi3, VOIperi5, and VOIperi10, and a total of 1206 features were extracted for each VOI. The inter- and intra-observer consistency test, Spearman’s rank correlation test, hypothesis test (Student’s t-test or Mann-Whitney U-test), and LASSO algorithm were used for radiomics feature selection in order. The logistic regression was used to construct radiomics model, with penalty parameter tuning conducted by 5-fold cross-validation, and the radscore was calculated. Our study constructed one intratumor radiomics model and three peritumoral radiomics models (3 mm peritumoral model, 5 mm peritumoral model, and 10 mm peritumoral model). Finally, we built intratumoral combined peritumoral radiomics model by combining the radiomics features of peritumoral radiomics model and intratumoral radiomics model. The intratumoral combined peritumoral radiomics models included intratumoral plus 3 mm peritumoral model, intratumoral plus 5 mm peritumoral model, and intratumoral plus 10 mm peritumoral model. The ROC analysis was used for the evaluation of model performance.Results
The radiomics models demonstrated favorable discrimination in the both cohorts (AUC: training 0.760 - 0.864, validation 0.742 - 0.873), and the intratumoral plus 3 mm peritumoral model showed the best predictive performance with the AUCs of 0.864(0.783 - 0.945) and 0.873(0.751 - 0.994) in the training and validation cohorts. ROC curves and discriminative performance of the seven radiomics models in the two cohorts are shown in Table 1.Discussion and Conclusion
In the present study, we selected the most significant predictive radiomics features (intratumoral, peritumoral expansion (3 mm, 5 mm and 10 mm), and intratumoral plus peritumoral expansion (3 mm, 5 mm and 10 mm)) from preoperative CE-MR images and developed radiomics models for the prediction. The intratumoral plus 3 mm peritumoral radiomics model showed the best predictive performance among the seven radiomics models. This might be interpreted that arterial peritumoral enhancement and irregular margin presented in the peritumoral area are independent predictors of prognosis in HCC patients. Therefore, the radiomics model with proper border extensions may contribute to improve prediction performance. In conclusion, the radiomics approach based on intratumoral and peritumoral regions has potential to improve performance for response prediction using preoperative CE-MRI. The accurate identification of HCC patients who would receive benefit from upfront TACE might potentially help decision-making for subsequent treatment strategies.Acknowledgements
NoneReferences
[1] Villanueva A. Hepatocellular Carcinoma. N Engl J Med 2019;380:1450-1462.
[2] Bruix J, Sala M, Llovet JM. Chemoembolization for hepatocellular carcinoma. Gastroenterology 2004;127:S179-188.
[3] Aerts HJ. The potential of radiomic-based phenotyping in precision medicine: a review. JAMA Oncol 2016;2:1636-1642.
[4] Shan QY, Hu HT, Feng ST, et al. CT-based peritumoral radiomics signatures to predict early recurrence in hepatocellular carcinoma after curative tumor resection or ablation. Cancer Imaging 2019;19:11.[5] Song W, Yu X, Guo D, et al. MRI-Based Radiomics: Associations With the Recurrence-Free Survival of Patients With Hepatocellular Carcinoma Treated With Conventional Transcatheter Arterial Chemoembolization. J Magn Reson Imaging 2020;52:461-473.
[6] Cheng Z, Yang P, Qu S, et al. Risk factors and management for early and late intrahepatic recurrence of solitary hepatocellular carcinoma after curative resection. HPB (Oxford) 2015;17:422-427.
[7] Zhu XD, Zhang JB, Zhuang PY, et al. High expression of macrophage colony-stimulating factor in peritumoral liver tissue is associated with poor survival after curative resection of hepatocellular carcinoma. J Clin Oncol 2008;26:2707-2716.
Figures