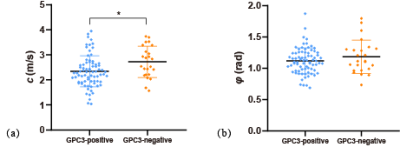4250
Multifrequency MR elastography for the detection of Glypican 3-positive hepatocellular carcinoma1Department of Radiology, Department of Radiology, Ruijin Hospital,Shanghai Jiao Tong University School of Medicine, Shanghai, China, 2Department of Radiology, Charité–Universitätsmedizin Berlin, Berlin, Germany, Berlin, Germany
Synopsis
Glypican-3 (GPC3) expression in hepatocellular carcinoma (HCC) is often associated with a poor prognosis. GPC3 is a promising target for tumor-specific immunotherapy in HCC. We investigated the diagnostic performance of viscoelastic properties quantified by tomoelastography, a multifrequency MR-elastography (MRE) technique, for the detection of GPC3-positive HCC. Preliminary results showed that reduced stiffness quantified by tomoelastography is a mechanical signature of positive GPC3 expression in HCC. Combining stiffness and serum alpha-fetoprotein (AFP) level could be considered as a viable biomarker for detecting GPC3-positive HCC as well as for predicting the outcome of GPC3-targeted immunotherapy.
Introduction
Hepatocellular carcinoma (HCC) is the most common primary malignant liver tumor and ranks as the third leading cause of cancer death worldwide[1]. Immunotherapy represents one promising treatment, however, there is still an urgent need to identify molecular targets and develop biomarkers to assess the therapeutic response[2]. Glypican-3 (GPC3), a member of heparan sulfate proteoglycans in the extracellular matrix (ECM), is highly expressed in >60% of HCCs and is associated with poorer prognosis[3-6]. These characteristics make GPC3 a potential immunotherapeutic target for HCC[7,8]. As GPC3 expression is still examined mainly through immunohistochemical analysis, a non-contrast-agent based quantitative imaging method for the detection of GPC3 is desirable. Tomoelastography, an advanced multifrequency MRE technique[9] provides shear wave speed (c in m/s, representing stiffness) and phase angle (φ in rad, relating to viscosity or tissue fluidity) that are sensitive to the structural and compositional changes of the ECM[10] due to disease. We hypothesized that biomechanical parameters could be indicative to GPC3 which mediates cell-ECM and cell-cell interactions. Therefore, we have investigated in this study the relationship between the biomechanical properties and GPC3 expression in patients with HCC.Methods
A total of 95 patients with 100 pathologically confirmed HCC lesions were included. Tomoelastography examinations were performed on a 1.5-Tesla MRI scanner (Magnetom Aera, Siemens, Germany) with four mechanical frequencies of 30, 40, 50, and 60 Hz. The setup and imaging sequence were similar to that described in Shahryari et.al[10]. In brief, 3D wave fields were acquired using a single-shot, spin-echo, echo-planar imaging sequence. Fifteen 5mm thick slices with 3×3mm3 in-plane resolution were acquired during free breathing. Liver Imaging Reporting and Data System (LI-RADS) categories and histopathologic were obtained for all patients. Qualitative and quantitative data between groups with different GPC3 expression were compared. Area-under-the-curve (AUC) analysis was performed to assess the diagnostic performance in detecting GPC3-positive HCC. We also analyzed the diagnostic performance of tomoelastography in detecting GPC3-positive HCC compared to serum alpha-fetoprotein (AFP), which is the most widely used biomarker for HCC screening and early diagnosis[11]. All statistical analyses were performed with SPSS version 26, GraphPad Prism 8.0 and MedCalc software.Results
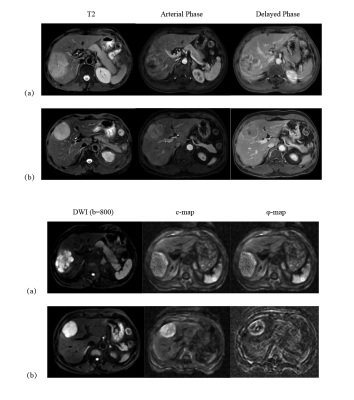
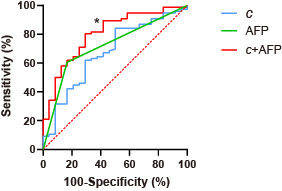
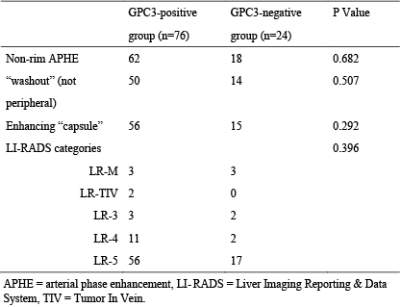
Based on histologic findings, the patients were divided into two groups: GPC3-positive (n=72) and GPC3-negative (n=23). In Figure 1, clinical imaging data for LIRADS stratification and tomoelastography c and φ maps for GPC3-positive (a) and GPC3-negative (b) HCC patients are shown. It is visible that the GPC3-postivie HCC is softer (lower c-value) than the GPC3-positive tumor. Comparing these two groups with different GPC3 expression, the occurrence of LI-RADS imaging features and the distribution of LI-RADS categories were similar (Table 1). Based on tomoelastography data, significantly lower c values were observed in the HCCs from the GPC3-positive group than from the GPC3-negative group (2.34±0.62 vs 2.72±0.62, P=0.010, Figure 2a). φ of the HCCs showed no sensitivity to the GPC3 expression (GPC3-possitive: 1.11±0.21 vs GPC3-negative: 1.18±0.27, P=0.214, Figure 2b). In terms of diagnostic performance, c detected GPC3-positive HCCs with an AUC of 0.67 (95% CI: 0.57-0.76, cutoff: 2.8 m/s) which was similar to the diagnostic performance of AFP level (AUC: 0.72, 95% CI: 0.62-0.80; cutoff: 20mg/L; P=0.57). Based on the AUC analysis, c and AFP demonstrated high sensitivity (84.2%) and specificity (83.3%), respectively. Therefore, combining c and AFP yielded significantly higher AUROC of 0.803 (95% CI: 0.711-0.876) compared with using either c (P=0.024) or APF (P=0.0065) alone (Figure 3).Discussion
In our patient cohort, we firstly established that the conventional LI-RADS categories used for imaging-based diagnostics of HCC were not sensitive to GPC3 expression, implying that tumor morphology and perfusion might not be directly related to GPC3 regulation.Biomechanical properties quantified by tomoelastography, on the other hand, demonstrated sensitivity to GPC3 expression. The unique softening feature obtained in the GPC3-positive HCC might be related to epithelial–mesenchymal transition (EMT), a process promoted by GPC3 expression[3]. Studies on cell biomechanics revealed that the metastatic cancer cells are often softer which facilitated the invasion through confinement and blood vessels[12-14]. Recently, it has also been reported that EMT softened cancer cells to migrate in matrix environments[15,16]. It is conceivable that the macroscopic softening of the GPC3-positive HCC as detect by tomoelastography reflects the collective behavior of soft and unjammed cancer cells due to GPC3-promted EMT[17].
Fluidity, another biomechanical parameter, showed no sensitivity of GPC3 expression. This is an unexpected result since GPC3 is known to upregulate cell motility making the tumor more fluid-like and resulting in elevated fluidity[18,19]. Considering that GPC3 is a heparan sulfate proteoglycans with negatively charged heparan sulfate chains, it is capable to bind large amounts of water. Therefore, overexpression of GPC3 on the HCC cell surface potentially reduces the amount of mobile water molecules thereby effectively turning the tissue into a more solid-like material. Thus, we hypothesized that the counteracting effects of cellular unjamming and water immobilization render the fluidity-related parameter φ less sensitive than stiffness for distinguishing GPC3-positive HCC.
Conclusion
Reduced stiffness quantified by tomoelastography is a mechanical signature of positive GPC3 expression in HCC. Combining stiffness and AFP level could be considered as a candidate for detecting GPC3-positive HCC. Collectively, our study lays the foundation for predicting HCC aggressiveness by MRE.Acknowledgements
No acknowledgement found.References
[1] Forner A, Reig M, Bruix J. Hepatocellular carcinoma[J]. Lancet, 2018, 391(10127): 1301-1314.
[2] Sangro B, Sarobe P, Hervas-Stubbs S, et al. Advances in immunotherapy for hepatocellular carcinoma[J]. Nat Rev Gastroenterol Hepatol, 2021, 18(8): 525-543.
[3] Zhou F, Shang W, Yu X, et al. Glypican-3: A promising biomarker for hepatocellular carcinoma diagnosis and treatment[J]. Med Res Rev, 2018, 38(2): 741-767.
[4] Ning S, Bin C, Na H, et al. Glypican-3, a novel prognostic marker of hepatocellular cancer, is related with postoperative metastasis and recurrence in hepatocellular cancer patients[J]. Mol Biol Rep, 2012, 39(1): 351-357.
[5] Fu SJ, Qi CY, Xiao WK, et al. Glypican-3 is a potential prognostic biomarker for hepatocellular carcinoma after curative resection[J]. Surgery, 2013, 154(3): 536-544.
[6] Xiao WK, Qi CY, Chen D, et al. Prognostic significance of glypican-3 in hepatocellular carcinoma: a meta-analysis[J]. BMC Cancer, 2014, 14: 104.
[7] Ho M, Kim H. Glypican-3: A new target for cancer immunotherapy[J]. European Journal of Cancer, 2011, 47(3): 333-338.
[8] Li N, Gao W, Zhang YF, et al. Glypicans as Cancer Therapeutic Targets[J]. Trends in Cancer, 2018, 4(11): 741-754.
[9] Tzschätzsch H, Guo J, Dittmann F, et al. Tomoelastography by multifrequency wave number recovery from time-harmonic propagating shear waves[J]. Med Image Anal, 2016, 30: 1-10.
[10] Shahryari M, Tzschätzsch H, Guo J, et al. Tomoelastography Distinguishes Noninvasively between Benign and Malignant Liver Lesions[J]. Cancer Res, 2019, 79(22): 5704-5710.
[11] Galle PR, Foerster F, Kudo M, et al. Biology and significance of alpha-fetoprotein in hepatocellular carcinoma[J]. Liver Int, 2019, 39(12): 2214-2229.
[12] Guck J, Schinkinger S, Lincoln B, et al. Optical deformability as an inherent cell marker for testing malignant transformation and metastatic competence[J]. Biophysical Journal, 2005, 88(5): 3689-3698.
[13] Wirtz D, Konstantopoulos K, Searson PC. The physics of cancer: the role of physical interactions and mechanical forces in metastasis[J]. Nat Rev Cancer, 2011, 11(7): 512-522.
[14] Rianna C, Radmacher M, Kumar S. Direct evidence that tumor cells soften when navigating confined spaces[J]. Molecular Biology of the Cell, 2020, 31(16): 1726-1734.
[15] Chen YQ, Lan HY, Wu YC, et al. Epithelial-mesenchymal transition softens head and neck cancer cells to facilitate migration in 3D environments[J]. J Cell Mol Med, 2018.
[16] Schrader J, Gordon-Walker TT, Aucott RL, et al. Matrix stiffness modulates proliferation, chemotherapeutic response, and dormancy in hepatocellular carcinoma cells[J]. Hepatology, 2011, 53(4): 1192-1205.
[17] Oswald L, Grosser S, Smith DM, et al. Jamming transitions in cancer[J]. J Phys D Appl Phys, 2017, 50(48): 483001.
[18] Asbach P, Ro SR, Aldoj N, et al. In Vivo Quantification of Water Diffusion, Stiffness, and Tissue Fluidity in Benign Prostatic Hyperplasia and Prostate Cancer[J]. Invest Radiol, 2020, 55(8): 524-530. [19] Li M, Guo J, Hu P, et al. Tomoelastography Based on Multifrequency MR Elastography for Prostate Cancer Detection: Comparison with Multiparametric MRI[J]. Radiology, 2021, 299(2): 362-370.
Figures